Method for synthesizing cadmium hydroxide single-crystal nanowire
A single-crystal nanometer and cadmium hydroxide technology, which is applied in chemical instruments and methods, cadmium compounds, inorganic chemistry, etc., can solve problems such as unfavorable large-scale industrial production, reduced purity of final products, and complicated reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
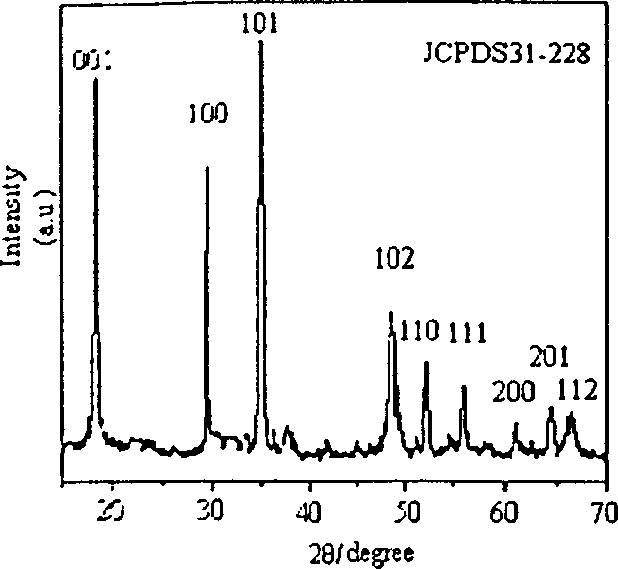
[0014] Weigh 0.001mol of analytically pure cadmium chloride and place it in a beaker, dissolve it in 20ml of deionized water, then adjust the pH value of the solution to 14 with 5% potassium hydroxide solution to obtain white Cd(OH) 2 Precipitation was continued and stirring was continued for 10 minutes. The white Cd(OH) obtained above 2 The precipitate was washed by centrifugation until the pH value was 7, and then the white Cd(OH) 2 The precipitate was redispersed in 18 ml of water, and 0.3 g of potassium chloride was added as a mineralizer. Transfer the above solution into a 20ml stainless steel pressure-resistant reaction kettle, react at 250°C for 12 hours, then cool to room temperature, filter the product with a Buchner funnel, wash with deionized water, and dry at 60°C to obtain a white Cd(OH) 2 powder. The product was identified as hexagonal cadmium hydroxide by X-ray powder diffraction; the morphology of the product was detected by TEM electron microscope: the dia...
Embodiment 2
[0016] Weigh 0.001mol of analytically pure cadmium chloride and place it in a beaker, dissolve it in 20ml of deionized water, then adjust the pH value of the solution to 14 with 5% potassium hydroxide solution to obtain white Cd(OH) 2 Precipitation was continued and stirring was continued for 10 minutes. The white Cd(OH) obtained above 2 The precipitate was washed by centrifugation until the pH value was 7, and then the white Cd(OH) 2 The precipitate was redispersed in 18 ml of water, and 0.3 g of sodium nitrate was added as a mineralizer. Transfer the above solution into a 20ml stainless steel pressure-resistant reaction kettle, react at 250°C for 12 hours, then cool to room temperature, filter the product with a Buchner funnel, wash with deionized water, and dry at 60°C to obtain a white Cd(OH) 2 powder. The product was detected by X-ray powder diffraction and TEM electron microscope.
Embodiment 3
[0018] Weigh 0.001mol of analytically pure cadmium nitrate and place it in a beaker, dissolve it in 20ml of deionized water, then adjust the pH value of the solution to 14 with 5% potassium hydroxide solution to obtain white Cd(OH) 2 Precipitation was continued and stirring was continued for 10 minutes. The white Cd(OH) obtained above 2 The precipitate was washed by centrifugation until the pH value was 7, and then the white Cd(OH) 2 The precipitate was redispersed in 18 ml of water, and 0.3 g of sodium sulfate was added as a mineralizer. Transfer the above solution into a 20ml stainless steel pressure-resistant reaction kettle, react at 250°C for 12 hours, then cool to room temperature, filter the product with a Buchner funnel, wash with deionized water, and dry at 60°C to obtain a white Cd(OH) 2 powder. The product was detected by X-ray powder diffraction and TEM electron microscope.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com