Process for preparing vinylidene chloride from rectifying residual liquid of dichloroethane
A technology for producing vinylidene chloride and vinylidene chloride is applied in the field of preparation of vinylidene chloride, which can solve problems such as environmental pollution and waste of raw materials, and achieve the effects of reducing the discharge of three wastes and achieving good social and economic benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
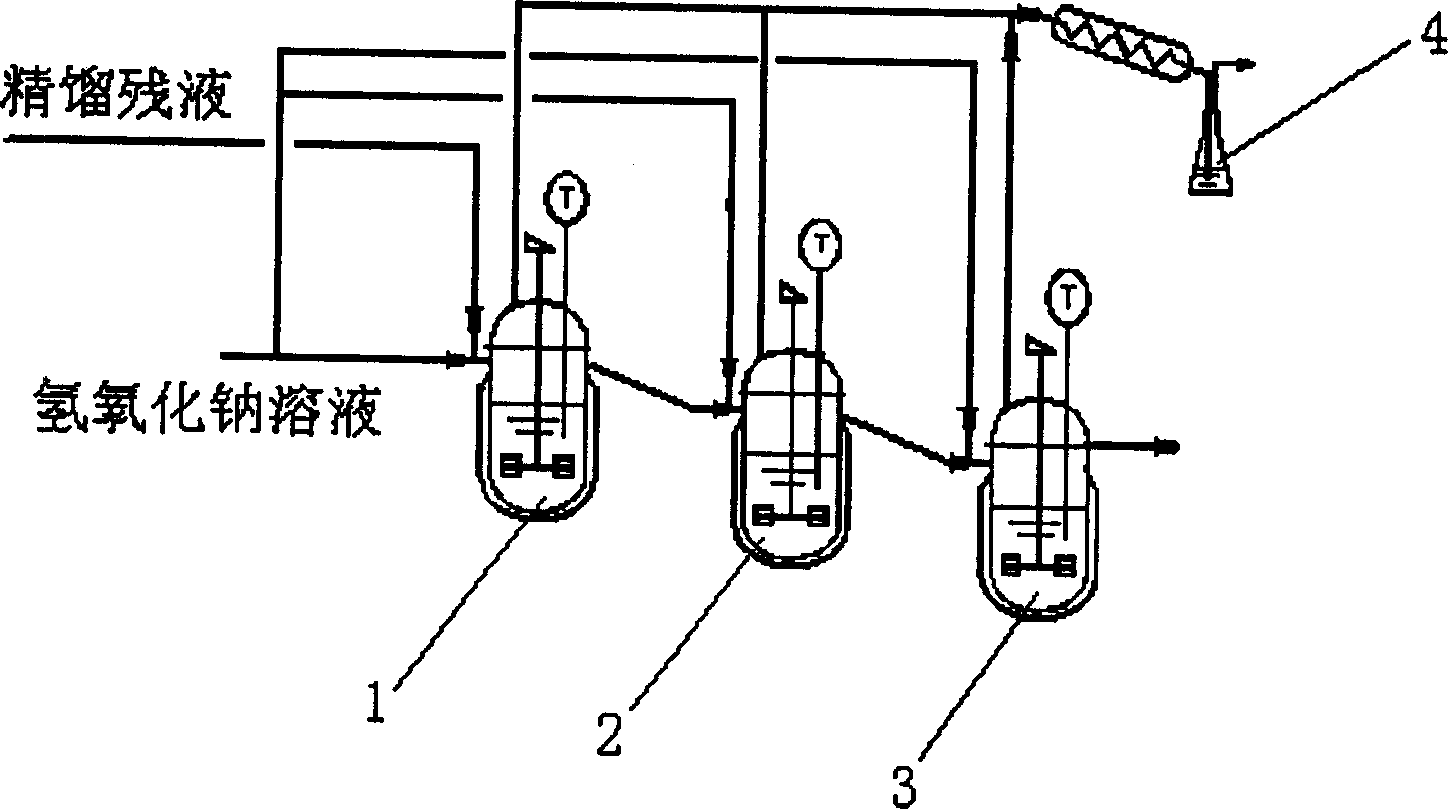
[0040] The rectification raffinate enters the first reactor, and flows into a plurality of reactors thereafter in sequence, while the sodium hydroxide solution with a weight concentration of 15% flows into the second reactor 2 and the third reactor 3, and the reaction temperature is 80°C. The residence time is 40 minutes, and the vapor phase products of multiple reactors enter the condenser liquid collector 4 connected therewith to collect vapor phase components such as vinylidene chloride.
[0041] Components and weight percentages of the rectification raffinate include:
[0042] 1,1,2-Trichloroethane 5% (wt), 1,2-Dichloroethane 80% (wt), 1,1,2,2-Tetrachloroethane 6% (wt), Pentachloro Ethane 7% (wt), polychloride 2% (wt);
[0043] In the first and second stills, the mol ratio of 1,1,2-trichloroethane and sodium hydroxide is 1: 0.2;
[0044] The molar ratio of 1,1,2-trichloroethane to sodium hydroxide in the third reaction kettle is 1:1.1.
[0045] The conversion rate reach...
Embodiment 2
[0047] Adopt the process condition and the device of embodiment 1, wherein, residence time is 30min, and the component and weight percent content of described rectification raffinate comprise:
[0048] 1,1,2-Trichloroethane 50% (wt), 1,2-Dichloroethane 40% (wt), 1,1,2,2-Tetrachloroethane 6% (wt), Pentachloro Ethane 2% (wt), polychloride 2% (wt)
[0049] 1,1,2-Trichloroethane 5% (wt), 1,2-Dichloroethane 80% (wt), 1,1,2,2-Tetrachloroethane 6% (wt), Pentachloro Ethane 7% (wt), polychloride 2% (wt);
[0050] In the first and second stills, the mol ratio of 1,1,2-trichloroethane and sodium hydroxide is 1: 0.9;
[0051] The molar ratio of 1,1,2-trichloroethane to sodium hydroxide in the third reaction kettle is 1:1.4.
[0052] The conversion rate reached 94.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com