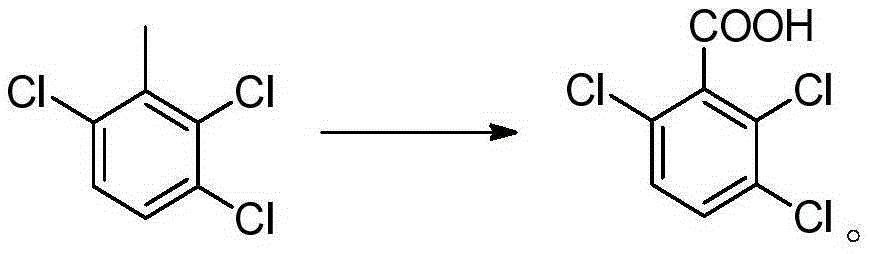
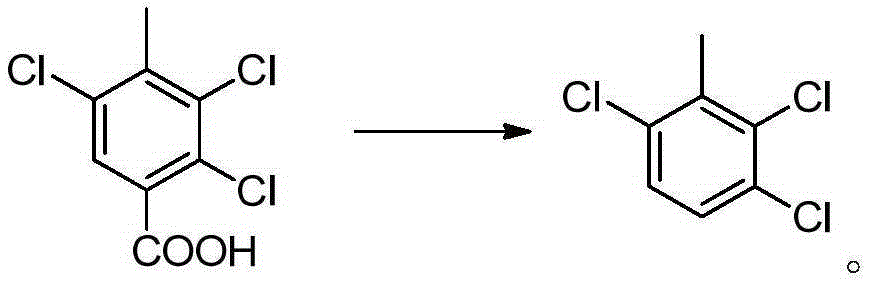
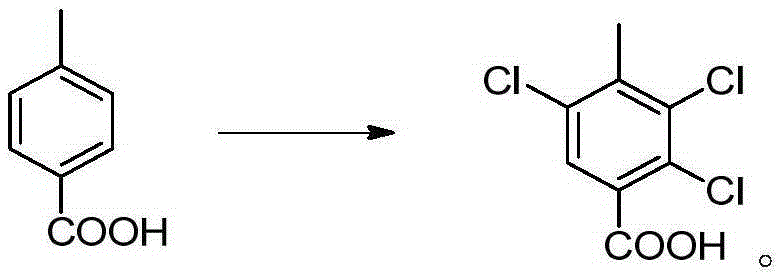
Synthesis methods of 3, 6-dichloro-2-methoxybenzoic acid and its intermediate
A technology of p-toluic acid and synthesis method, which is applied in the directions of oxidative preparation of carboxylic acid, chemical instruments and methods, and ozonation of carboxylic acid to prepare carboxylic acid, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0081] Embodiment 12,3, the preparation of 5-trichloro-p-methylbenzoic acid
[0082] Add 68g of p-toluic acid (HPLC purity 99%) to 200g of methanesulfonic acid, add 0.81g of ferric chloride, heat to 80°C and stir, and slowly feed 106.5g of chlorine gas under normal pressure (101325Pa). Reacted for 10 hours, the liquid phase (HPLC) detected that the area content of 2,3,5-trichloro-p-methylbenzoic acid was about 50%, and then cooled to room temperature, and the white crystals separated out by suction filtration were washed with a small amount of methanesulfonic acid, and 50 g of acetic acid was added Recrystallize, filter after cooling, mainly monochlorine product and dichloroproduct in the mother liquor, apply mechanically to next batch of chlorination reaction, filter cake is 2,3,5-trichloro-p-methylbenzoic acid 64.7g, HPLC purity is 93%, yield 54%, yield 85% after applying mechanically.
Embodiment 22
[0083] Embodiment 22,3, the preparation of 5-trichloro-p-methylbenzoic acid
[0084] Add 68g of p-toluic acid (HPLC purity 99%) to 200g of methanesulfonic acid, add 0.81g of ferric chloride, heat to 120°C and stir, and slowly feed 106.5g of chlorine gas under normal pressure (101325Pa). Reacted for 6 hours, the liquid phase (HPLC) detected that the area content of 2,3,5-trichloro-p-methylbenzoic acid was about 50%, and then cooled to room temperature, and the white crystals separated out by suction filtration were washed with a small amount of methanesulfonic acid, and 50 g of acetic acid was added Recrystallization, filter after cooling, mainly monochlorine product and dichloroproduct in the mother liquor, apply mechanically to next batch of chlorination reaction, filter cake is 2,3,57.5g of 5-trichloro-p-toluic acid, HPLC purity is 91%, 48% yield, 80% yield after applying mechanically.
Embodiment 32
[0085] Embodiment 32,3, the preparation of 5-trichloro-p-methylbenzoic acid
[0086] Add 68g of p-toluic acid (HPLC purity 99%) to 200g of methanesulfonic acid, add 0.81g of ferric chloride, heat to 50°C and stir, and slowly feed 106.5g of chlorine gas under normal pressure (101325Pa). After 24 hours of reaction, the area content of 2,3,5-trichloro-p-toluic acid was detected by liquid phase (HPLC) to be about 50%, and then it was lowered to room temperature, and the white crystals separated out were filtered by suction, washed with a small amount of methanesulfonic acid, and 50 g of acetic acid was added to Crystallized, filtered after cooling, mainly monochlorine product and dichlorine product in the mother liquor, applied mechanically to the next batch of chlorination reaction, the filter cake was 69.4g of 2,3,5-trichloro-p-methylbenzoic acid, and the HPLC purity was 94 %, the yield is 58%, and the yield is 87% after applying mechanically.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com