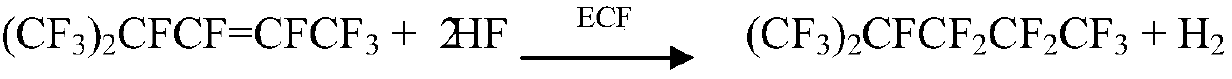Preparation method of perfluorohexane
A fluorohexane and electrolytic fluorination technology, applied in the field of fluoroalkanes, can solve the problems of increased leakage risk of hydrogen fluoride, high pressure of the electrolytic fluorination system, and increased difficulty in purification and separation, etc. Long cycle and the effect of maintaining concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Add 35kg of hexafluoropropylene dimer, 65kg of AHF and 1.0kg of sodium fluoride into the electrolyte preparation tank respectively, stir at 5°C and 50rpm for 30min to obtain the electrolyte, and set aside;
[0033] (2) Put the electrolyte obtained in step (1) into the water removal tank for electrolytic dehydration. The initial current of electrolytic dehydration is 10A. After electrolytic dehydration starts, the current is increased at a rate of 10A / 2h. When the current reaches 60A When the electrolytic dehydration is finished, the electrolyte solution after dehydration is obtained for subsequent use;
[0034] (3) The dewatered electrolyte solution obtained in step (2) is continuously added to the electrolytic cell (the anode material is 6# nickel, and the cathode material is carbon steel), and the electrolytic fluorination reaction is carried out at a temperature of 19°C under normal pressure , the electrolysis voltage is controlled to be 6V, and the working curre...
Embodiment 2
[0037] (1) Add 10kg of hexafluoropropylene dimer, 50kg of AHF and 0.5kg of sodium fluoride into the electrolyte preparation tank respectively, stir at 15°C and 80rpm for 5min to obtain the electrolyte, and set aside;
[0038] (2) Put the electrolyte obtained in step (1) into the water removal tank for electrolytic dehydration. The initial current of electrolytic dehydration is 10A. After electrolytic dehydration starts, the current is increased at a rate of 5A / 2h. When the current reaches 50A When the electrolytic dehydration is finished, the electrolyte solution after dehydration is obtained for subsequent use;
[0039] (3) The electrolyte obtained in step (2) after water removal is continuously added to the electrolytic cell (the anode material is 6# nickel, and the cathode material is carbon steel), and the electrolytic fluorination reaction is carried out at a temperature of 20 ° C and normal pressure , control the electrolysis voltage at 5V, working current 60A, the gas pha...
Embodiment 3
[0042] (1) Add 50kg of hexafluoropropylene dimer, 80kg of AHF and 0.3kg of sodium fluoride into the electrolyte preparation tank respectively, stir at 10°C and 70rpm for 10min to obtain the electrolyte, and set aside;
[0043] (2) Put the electrolyte obtained in step (1) into the water removal tank for electrolytic dehydration. The initial current of electrolytic dehydration is 8A. After electrolytic dehydration starts, the current is increased at a rate of 7A / 2h. When the current reaches 52A When the electrolytic dehydration is finished, the electrolyte solution after dehydration is obtained for subsequent use;
[0044] (3) The electrolyte obtained in step (2) after water removal is continuously added to the electrolytic cell (the anode material is 6# nickel, and the cathode material is carbon steel), and the electrolytic fluorination reaction is carried out at a temperature of 15°C under normal pressure , control the electrolysis voltage at 7V, working current 30A, the gas p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com