Compounds and methods for treatment of asthma, allergy and inflammatory disorders
A compound, an independent technology, applied in the direction of active ingredients of heterocyclic compounds, allergic diseases, anti-inflammatory agents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
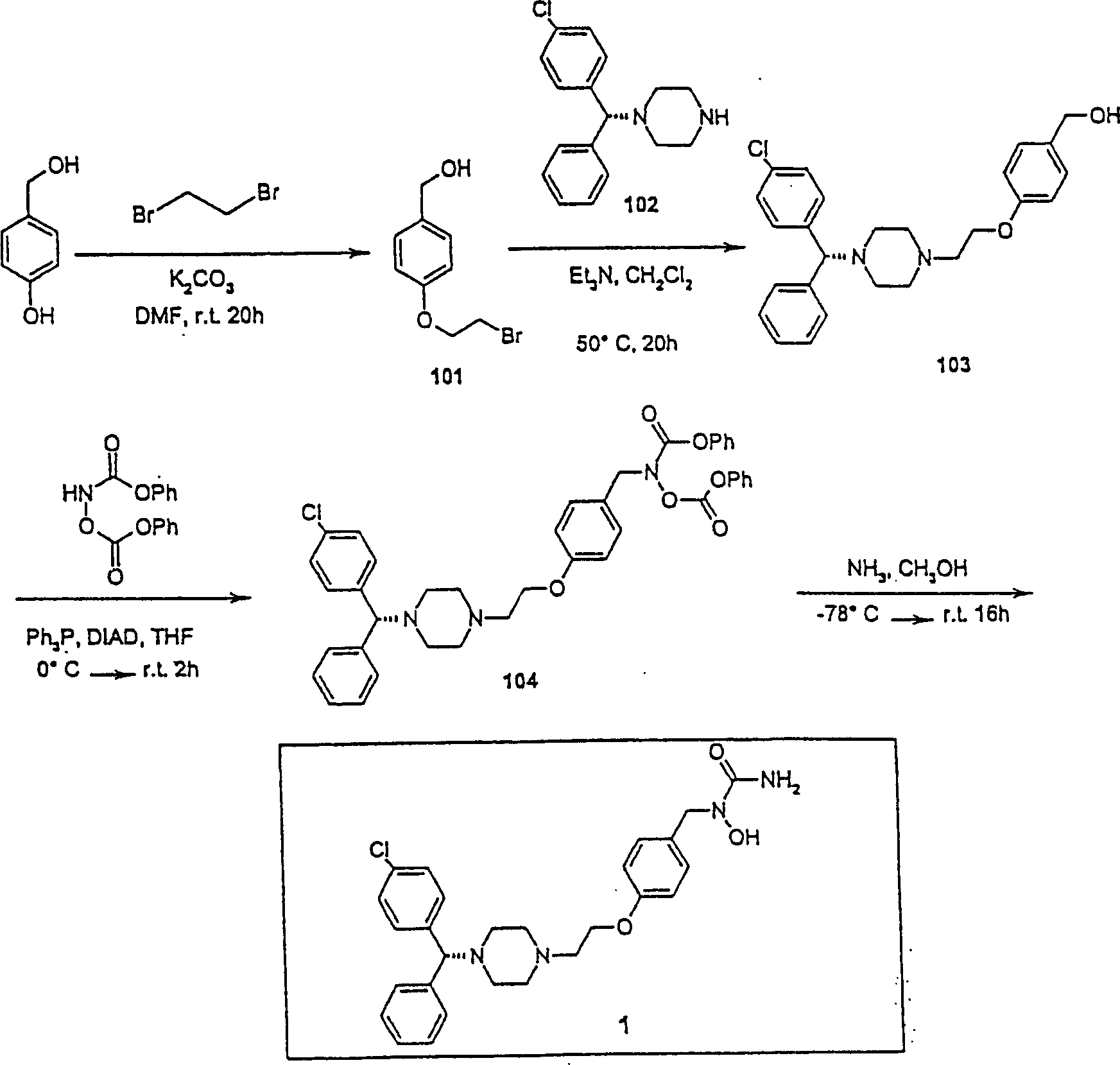
[0135] N-{[4-(2-{4-[(1R)(4-chlorophenyl)phenylmethyl]piperazinyl}ethoxy)phenyl]methyl}-amino-N-hydroxylamide ( Compound 1, figure 1 ) preparation
[0136] 4-(2-Bromoethoxy)benzyl alcohol (compound 101)
[0137] To a solution of 4-hydroxybenzyl alcohol (2.0 g, 16.11 mmol) in DMF (10 mL) was added potassium carbonate (2.67 g, 19.32 mmol) and the reaction mixture was stirred at room temperature for 30 minutes before adding 1,2-dibromoethane (3.03 g, 16.13 mmol), the reaction mixture was stirred at room temperature for another 20 hours, then quenched with water and extracted with ethyl acetate. The organic layer was washed with water and brine and evaporated to give an oil, the residue was purified by flash column chromatography (silica gel, 3:1 hexane / ethyl acetate) to give compound 101 (1.7 g, 45.7%):
[0138] 1 H NMR (CDCl 3 ) δ 3.64 (t, 2H), 4.29 (t, 2H), 4.62 (s, 2H), 6.91 (d, 2H), 7.30 (d, 2H).
[0139] 4-{2-[4-((1R)(4-chlorophenyl)phenylmethyl)piperazinyl]ethoxy}b...
Embodiment 2
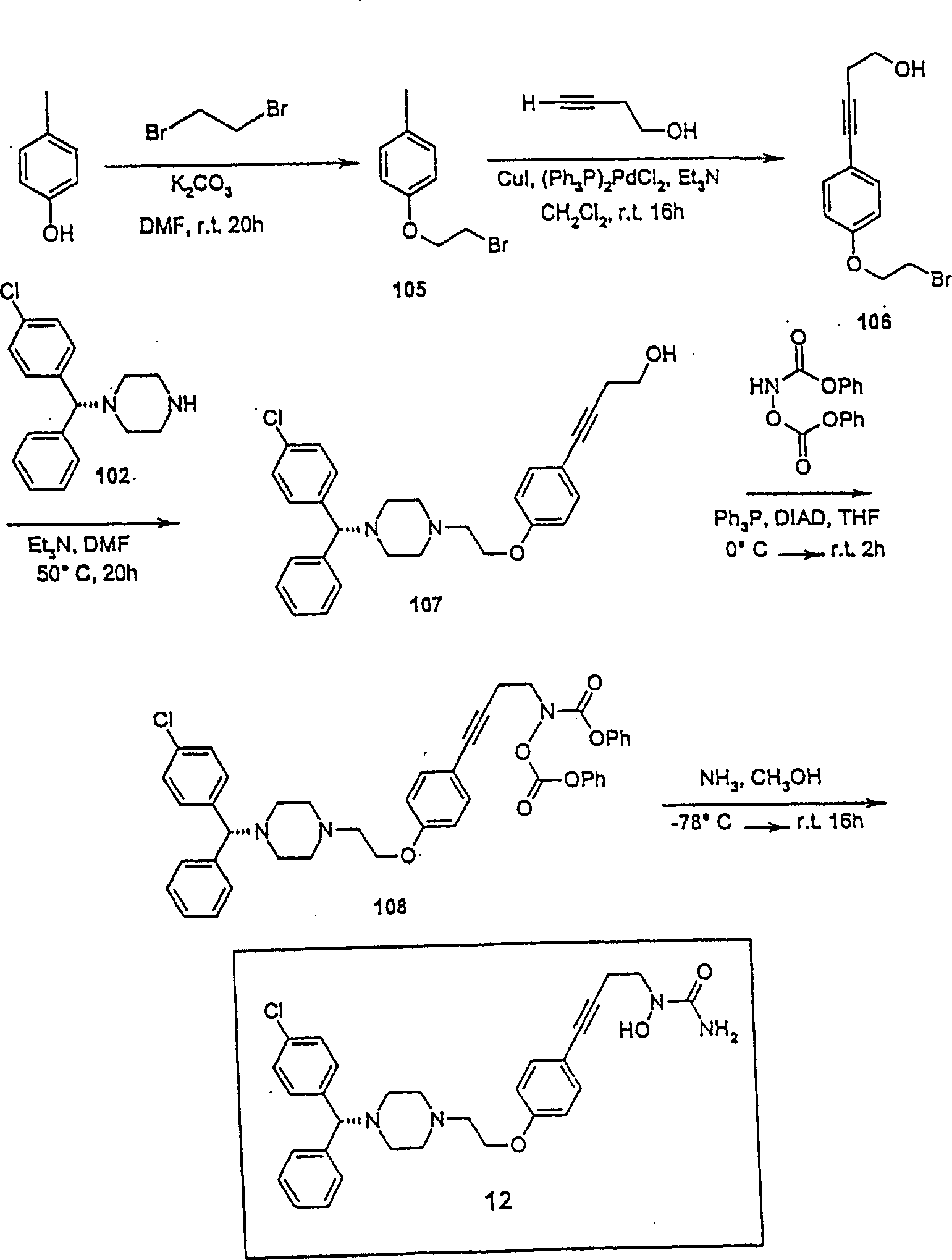
[0148] N-{4-[4-(2-{4-[(1R)(4-chlorophenyl)phenylmethyl]piperazinyl}ethoxy)phenyl]but-3-ynyl}-amino -N-hydroxyamide (compound 12, figure 2 ) preparation
[0149] 4-(2-Bromoethoxy)-1-iodobenzene (compound 105)
[0150] To a solution of 4-iodophenol (10.0 g, 45.45 mmol) in DMF (50 mL) was added potassium carbonate (12.6 g, 91.17 mmol), the reaction mixture was stirred at room temperature for 30 minutes, then 1,2-dibromoethane was added (17.07 g, 90.91 mmol), the reaction mixture was stirred at room temperature for an additional 16 hours, then quenched with water and extracted with dichloromethane. The organic layer was washed with water and brine and evaporated to give an oil, the residue was purified by flash column chromatography (silica gel, hexanes) to give compound 105 (2.7 g, 18.2%):
[0151] 1 H NMR (CDCl 3 ) δ 3.63 (t, 2H), 4.26 (t, 2H), 6.70 (d, 2H), 7.58 (d, 2H).
[0152] 4-[4-(2-Bromoethoxy)phenyl]but-3-yn-1-ol (Compound 106)
[0153] To compound 105 (2.7g,...
Embodiment 3
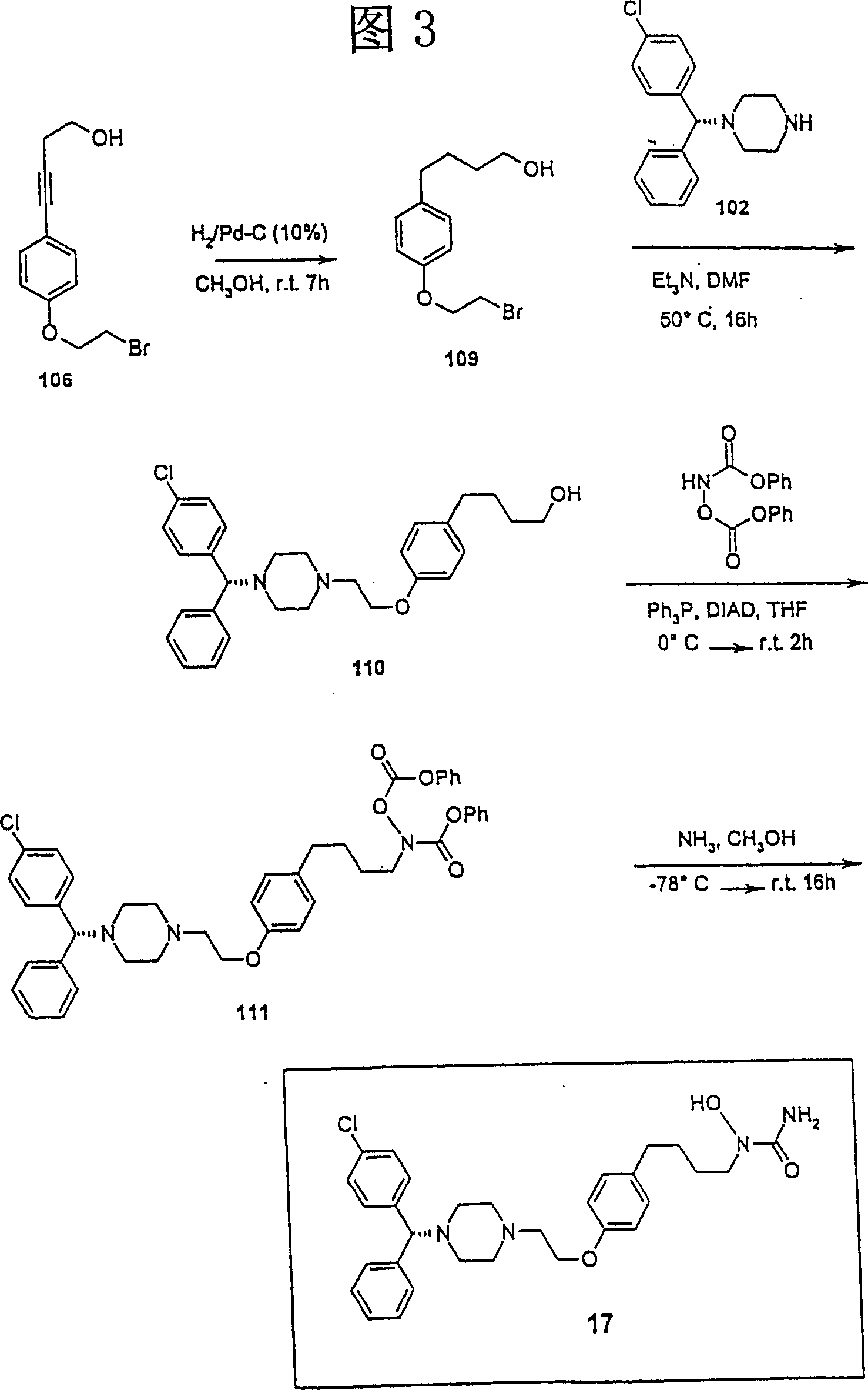
[0163] N-{4-[4-(2-{4-[(1R)(4-chlorophenyl)phenylmethyl]piperazinyl}ethoxy)phenyl]butyl}-amino-N-hydroxy Preparation of Amide (Compound 17, Figure 3)
[0164] 4-[4-(2-Bromoethoxy)phenyl]butan-1-ol (compound 109)
[0165] A solution of compound 106 (1.3 g, 4.83 mmol) in methanol (15 mL) was hydrogenated over 10% palladium / charcoal (130 mg) under balloon pressure for 7 hours, the catalyst was filtered off and the filtrate was evaporated to give compound 109 (1.31 g, 99.2% ): 1 H NMR (CDCl 3 ) δ 1.65 (m, 4H), 2.60 (t, 2H), 3.66 (m, 4H), 4.28 (m, 2H), 6.83 (d, 2H), 7.10 (d, 2H).
[0166] 4-{4-[2-(4-((1R)(4-chlorophenyl)phenylmethyl)piperazinyl)ethoxy]phenyl}butan-1- Alcohol (compound 110)
[0167] To a solution of compound 109 (1.3 g, 4.76 mmol) and [(1R)(4-chlorophenyl)phenylmethyl]-piperazine (102) (1.39 g, 4.86 mmol) in DMF (12 mL) was added triethyl Amine (762.3 mg, 7.55 mmol). The reaction mixture was stirred at 50°C for 16 hours, water was added and the reaction m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com