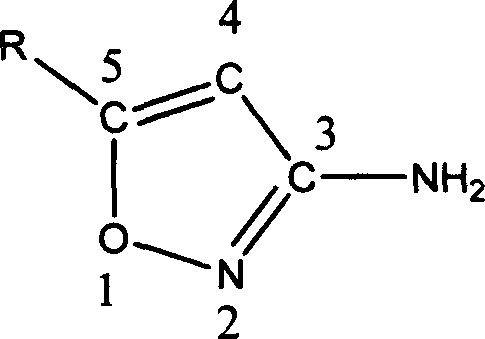
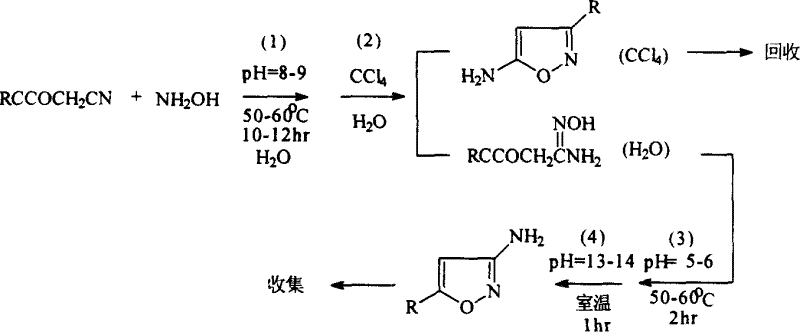
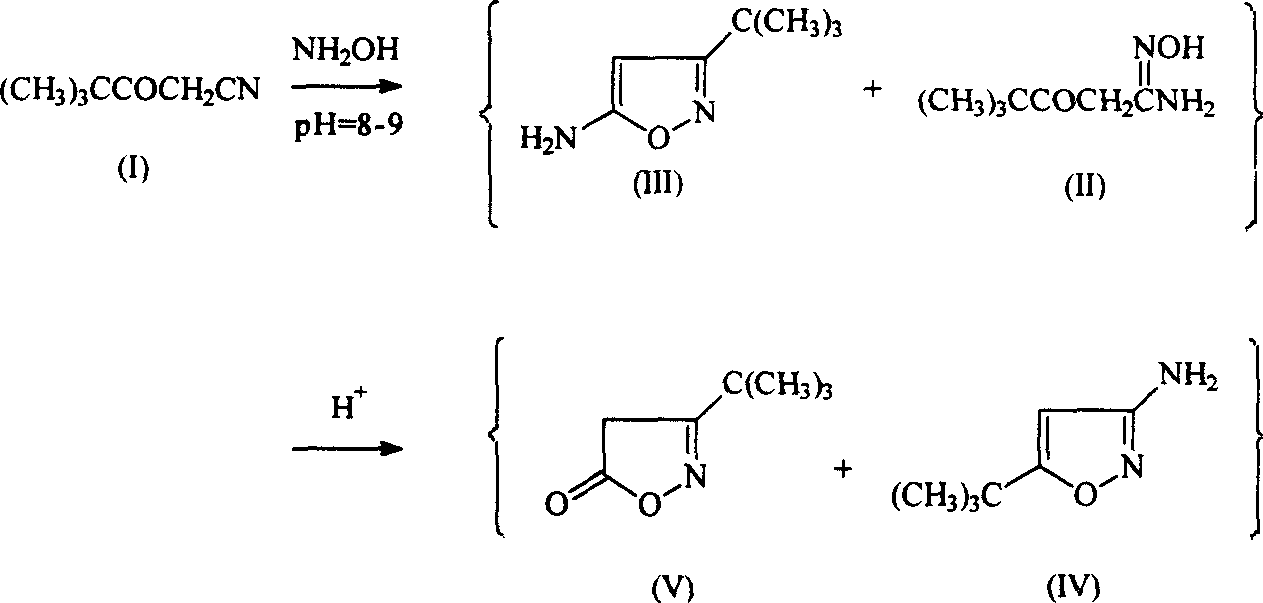
Method for preparing compound of 5-substitution-3-amido isoxazole
A technology for isoxazole compounds and aminoisoxazoles is applied in the field of preparation of 5-substituted-3-aminoisoxazole compounds, which can solve the problem of increasing difficulty in separation and purification of target products, affecting promotion and use, and reducing the yield of target products. and other problems, to achieve the effects of easy temperature control, increased yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the synthesis of 5-methyl-3-aminoisoxazole compound
[0029] Include the following steps:
[0030] Weigh the material by the following weight percentages: the weight percentages of methyl acetoacetonitrile, sodium hydroxide, hydroxylamine hydrochloride and water are respectively: 6.2%: 3.2%: 4.6%: 76%, the reaction temperature of control system is 50- 55°C;
[0031] Add 15ml of CCl to the above reaction product 4 , and then transferred to a 150ml separatory funnel, shaken for extraction, left to stand for layers, the lower layer of CCl 4 Layer is a yellowish brown liquid, the upper water layer is a light yellow liquid, reclaim CCl 4 Layer, collect the water layer in the reactor;
[0032] Adjust the pH value of the above collected solution to 5.0-6.0, adjust the temperature at 50-55°C, stir and react for 2 hours, after a small amount of crystal grains are precipitated, cool and stand still;
[0033] Adjust the pH value to 9-13, a large amount of prec...
Embodiment 2
[0035] Embodiment 2: Synthesis of 5-tert-butyl-3-aminoisoxazole compound
[0036] Include the following steps:
[0037] Weigh the material by the following weight percentages: the weight percentages of tert-butylacetoacetonitrile, sodium hydroxide, hydroxylamine hydrochloride and water are respectively: 7.5%: 2.7%: 4.6%: 85.2%, the reaction temperature of control system is 50 -60°C;
[0038] step is the same as in example 1, the difference is that CCl 4 Change the volume to 20ml;
[0039] Adjust the pH value of the above collected solution to 5.0-5.5, adjust the temperature at 50-60°C, stir and react for about 1 hr, a small amount of crystal grains precipitate, cool and stand still;
[0040] Adjust the pH value to 8-12, and a large amount of precipitates are formed. After filtering, washing and drying, a light yellow crystalline solid is obtained.
[0041] Melting point: 108-110°C; yield 82%
Embodiment 3
[0042] Embodiment 3: Synthesis of 5-methyl-3-aminoisoxazole compound
[0043] Include the following steps:
[0044] weighing material by following weight percentage: the percentage by weight of methyl acetoacetonitrile, potassium hydroxide, hydroxylammonium sulfate and water is respectively: 6.5%: 3.0%: 5.5%: 85%, the reaction temperature of control system is 45- 55°C;
[0045] step is the same as in example 1, the difference is that CCl 4 Change the volume to 10ml;
[0046] Adjust the pH value of the above collected solution to 4.5-5.0, adjust the temperature at 45-55°C, stir and react for 1.5hr, a small amount of crystal grains precipitate, cool and stand;
[0047] Adjust the pH value to 10-12, and a large amount of precipitates are formed. After filtering, washing and drying, a light yellow crystalline solid is obtained.
[0048] Yield 80%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com