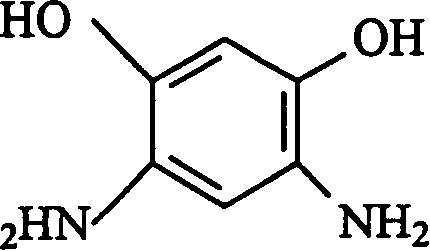
Process for preparing 4, 6-diamino resorcin
A technology of diaminoresorcinol and dihydroxybenzene, which is applied in 4 fields, can solve the problems that rare metal catalysts cannot be recycled economically, and achieve the prospect of large-scale industrial production, the effect of catalyst activity not being reduced, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Grind coconut shell activated carbon and sieve to obtain 350 mesh activated carbon powder, add a certain amount of activated carbon to 10% nitric acid, stir in 80°C water bath for 2 hours, then filter, wash with distilled water to become neutral; then steam Process, dry, and reserve.
[0025] Dissolve copper nitrate in deionized water to make 2.1×10 -1 mol / L copper nitrate solution, add 10g of activated carbon treated above to 37ml copper nitrate aqueous solution, soak for 2h, age in air for 24h, dry in air at 100-110°C for 24h, and bake in muffle furnace at 250°C for 5h , that is, to obtain 5% (wt) CuO / C for use. Dissolve chloroplatinic acid in deionized water to make 1.7×10 -2 mol / L chloroplatinic acid solution. Add activated carbon loaded with Cu to 278ml of chloroplatinic acid aqueous solution, soak for 2 hours, then add 8ml of formaldehyde for reduction, and at the same time add saturated sodium bicarbonate solution dropwise, control the pH value to about 9, rea...
Embodiment 2
[0029] The preparation of 3% CuO / C is the same as in Example 1. Dissolve palladium chloride in deionized water to make a 1.7×10 -2 mol / L palladium chloride solution. Add activated carbon loaded with Cu to 278ml of palladium chloride aqueous solution, soak for 2 hours, then add 8ml of formaldehyde for reduction, and at the same time add saturated sodium bicarbonate solution dropwise, control the pH value to about 9, react until the pH value remains unchanged, filter After drying, a Pd-Cu / C catalyst with a Pd content of 5% is obtained.
[0030] Add 70 g of 4,6-dinitro-2-chloro-1,3-dihydroxybenzene, 350 ml of propanol, and 7 g of the above 5% Pd-3% Cu / C into a 1000 ml reactor. Will N 2 Introduce air into this sealed reactor for three times, and then use H 2 Replace three times. h 2 The pressure reaches 3Mpa and the temperature reaches 80°C. After the reaction, the reaction feed liquid was cooled to room temperature, filtered, and the catalyst was left in the reactor for re...
Embodiment 3
[0033] The processing of activated carbon is as described in Example 1. Dissolve cobalt nitrate in deionized water to make 2.1×10 -1 mol / L cobalt nitrate solution, add 10g of activated carbon to 39ml cobalt nitrate aqueous solution, impregnate for 2h, age in air for 24h, dry in air at 100-110°C for 24h, and bake in muffle furnace at 250°C for 5h , that is, to obtain 5% (wt) CoO / C for use. According to the Pt loading method in Example 1, a 5%Pt-5%Co / C catalyst was prepared.
[0034] Add 70g of 4,6-dinitro-2-chloro-1,3-dihydroxybenzene, 350ml of benzene, and 7g of 5%Pt-5%Co / C into a 1000ml reactor. Will N 2 Introduce air into this sealed reactor for three times, and then use H 2 Replace three times. h 2 The pressure reaches 0.9Mpa and the temperature reaches 30°C. After the reaction, the reaction feed liquid was cooled to room temperature, filtered, and the catalyst was left in the reactor for reuse. The filtrate contained 4,6-diaminoresorcinol. According to liquid chroma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com