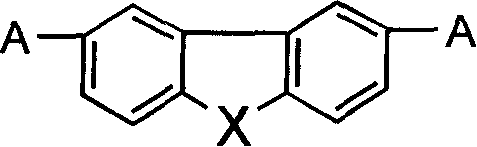
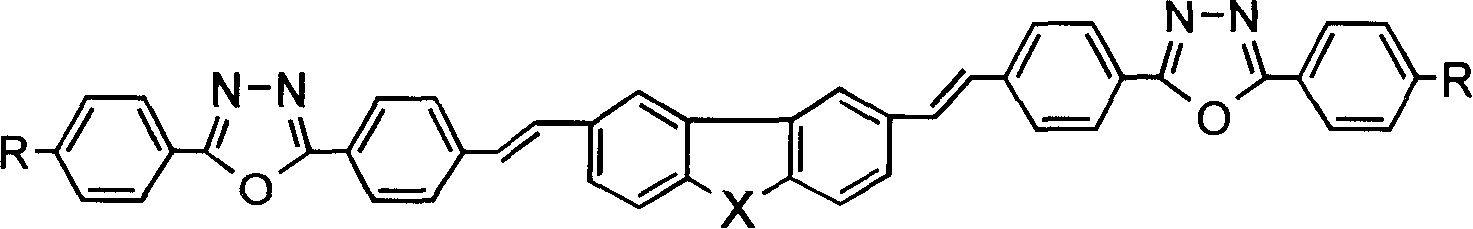
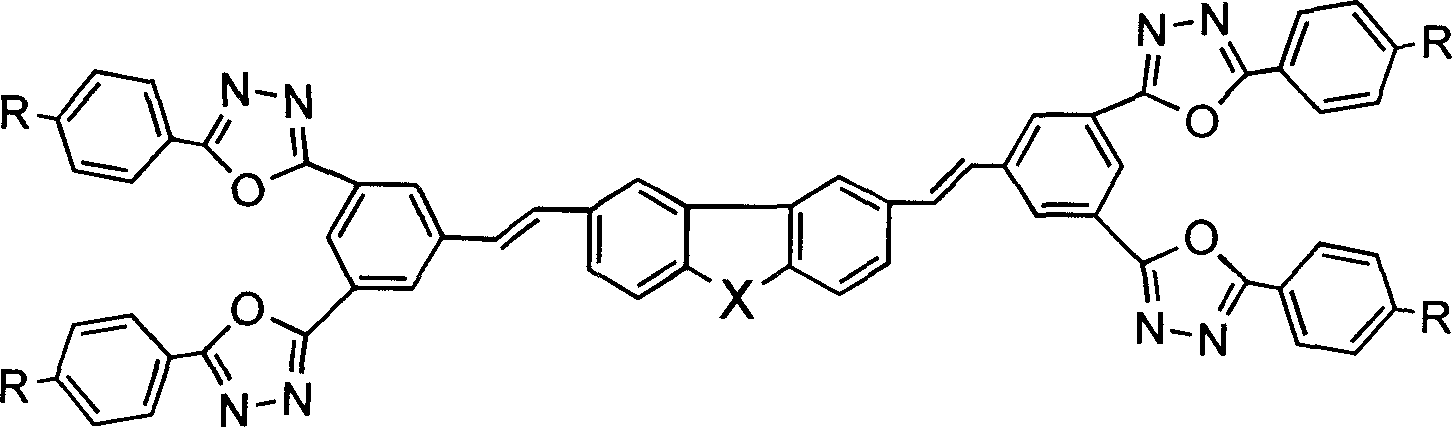
Heterofluorene derivative with strong two photon absorption character
A technology of two-photon absorption and derivatives, applied in organic chemistry and other fields, can solve the problems of high price and difficult synthesis of thiophene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: 2,7-bis[(2-4'-ethoxyphenyl-5-4'-styryl)-1,3,4-oxadiazole]thiafluorene and its synthesis.
[0031] (1) Add 40.0g (0.206mol) of homemade ethyl 4-ethoxybenzoate, 30ml of hydrazine hydrate (30.9g, 0.617mol), and 200ml of absolute ethanol into a single-necked round-bottomed flask in turn, and reflux for 1- 4 days. The solvent was distilled off, and 20 g of white solid 4-ethoxybenzohydrazide (a) was obtained by suction filtration, mp: 125-128°C.
[0032] (2) Compound (a), pyridine, and tetrahydrofuran were mixed in a beaker. The mixed solution was slowly added into 15.4 g of 4-methylbenzoyl chloride under stirring, and a white precipitate was formed immediately. After stirring at room temperature for several hours, it was slowly poured into ice water, allowed to stand overnight, filtered, and recrystallized from absolute ethanol to obtain 8.3 g of bishydrazide intermediate (b).
[0033] (3) Add POCl in turn to the round bottom flask 3 and intermediate (b), N 2...
Embodiment 2 to Embodiment 21
[0041] Example 2 to Example 21: The synthesis method is similar to Example 1, only need to change the 4-ethoxy ethyl benzoate in step (1) and the 2,7- Thiofluorenedial.
[0042] The general formula of the compound is,
[0043]
[0044] Example
R
X
Example 2
C(CH 3 ) 3
S
Example 3
F
S
Example 4
OC 2 h 5
O
Example 5
C(CH 3 ) 3
O
Example 6
F
O
Example 7
OC 2 h 5
NC 2 h 5
Example 8
OC 2 h 5
NC 4 h 9
Example 9
OC 2 h 5
NC 8 h 17
Example 10
OC 2 h 5
NC 12 h 25
Example 11
OC 2 h 5
NC 22 h 45
Example 12
C(CH 3 ) 3
NC 2 h 5
Example 13
C(CH 3 ) 3
NC 4 h 9
Example 14
C(CH 3 ) 3
NC 8 h 17
Example 15
C(CH 3 ) 3
NC 12 h 25
Example 16
C(CH 3 ) 3
NC 2...
Embodiment 22
[0048] Embodiment 22: Prepared according to the following steps,
[0049] (1) Dissolve 1-cyano-3,5-benzenedicarboxylic acid in 50ml SOCl 2 10ml of pyridine was added dropwise, and refluxed at 55°C for 7 hours to obtain a dark brown transparent liquid. Excess SOCl 2 and pyridine were distilled off under reduced pressure to obtain 1,3-diacyl chloride benzocyanide as a yellow solid.
[0050] (2) 9.36g (0.052mol) of 4-ethoxybenzohydrazide was dissolved in a mixture of THF and pyridine, then added dropwise to 1,3-diacylchloride benzocyanide, stirred at room temperature for 4 to 6 hours, The THF was distilled off under reduced pressure, the remaining liquid was poured into water, and a white solid precipitated after standing for 2 hours, filtered by suction and washed with ethanol to obtain 8 g of intermediate product (I), with a yield of 66%. M.p.252-255°C. 10g (0.021mol) of intermediate product (I) was dissolved in 125ml POCl 3 In, the reaction solution in N 2 It was refluxe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com