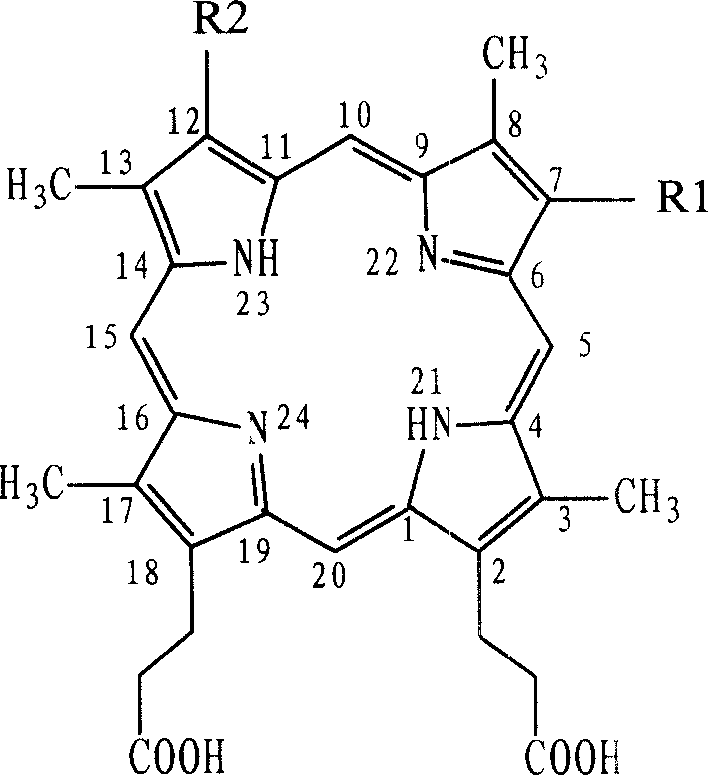
Application of haematopophyrin in treating ophthalmic disease
A technology of hematoporphyrin monomethyl ether and ophthalmic diseases, applied in the field of medicine, can solve the problems of unsatisfactory treatment effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: Preparation of hematoporphyrin monomethyl ether and therapeutic agent
[0057] A. Preparation of Hematoporphyrin Monomethyl Ether
[0058] By the method described in the Chinese patent application of CN 01131939, with 20 grams of 3,8-two (1-bromoethyl) hypoporphyrin IX hydrobromide and 3000 milliliters of methanol / water mixed solution stirring reaction 2 hours, The pH of the reaction solution was adjusted to 13 with 10 mol / L sodium hydroxide, stirred for 4 hours, and then the pH of the reaction solution was adjusted to 4-5 with glacial acetic acid to precipitate out. The separated precipitate was collected by Buchner funnel, washed with water and dried to obtain 19.5 g of crude product. Purify through silica gel column chromatography to obtain 3.9 grams of hematoporphyrin monomethyl ether ( figure 1 ).
[0059] Repeat the above steps to obtain about 20 grams of hematoporphyrin monomethyl ether.
[0060] B. Preparation of Therapeutic Agents
[0061] Ta...
Embodiment 2
[0062] Embodiment 2: CAM model is used for photodynamic therapy effect determination in vivo
[0063] The establishment of the CAM model adopts the following method: 37 ℃ incubator, the air chamber is upward, rotate 3 to 4 times a day, until the 9th day of hatching, disinfect the surface of the egg and make a small hole of 1 to 2 mm on the top of the air chamber, and place it at a distance from the fetal head. Mark a rectangular area of 1.0 cm × 1.5 cm on the projected part of the egg shell in the first 1 cm and between the two anterior yolk veins, grind and cut through the egg shell, and gently scratch a small hole with a diameter of about 1 mm on the egg shell membrane. A little sterile pure water was added dropwise to separate the eggshell membrane at the edge of the small hole, and a sterile microporous filter membrane carrier with a diameter of 6 mm was placed on the part of the CAM where the blood vessels were least.
[0064] Then 10 ul of hematoporphyrin monomethyl et...
Embodiment 3
[0066] Embodiment 3: the establishment of CNV mouse model
[0067] The CNV model was established as follows: randomly select 20 adult male C57BL-6J mice with a body weight of 25g-26g. Mice were anesthetized by intraperitoneal injection of 200 μL of 0.3% pentobarbital sodium, 2% tropicamide and 10% phenylephrine to dilate the pupils. Laser light (wavelength 810nm, diameter 75μm, irradiation time 0.1s, power 140mW) was introduced into the eyes of mice through a slit lamp and a contact lens. Fundus fluorescence angiography (FFA) was performed 1 week after laser irradiation, and the photocoagulation point was selected without fluorescence leakage, and the photocoagulation point was milky white (such as Figure 4 ) of 9 animals, the tissue section was observed to confirm the membrane formation or further photodynamic therapy test was carried out.
[0068] One of the above-mentioned animals was killed by excessive sodium pentobarbital, the eyeballs were removed, fixed in 2% glutar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com