Method and use of closed hydrophilic and lipophilic liquid-phase hollow capsules with cores
A self-sealing, water-lipophilic technology, applied in emulsion delivery, microcapsule preparation, microsphere preparation, etc., can solve the problems of expensive freeze-drying equipment, limited dissolved oxygen concentration, and decreased dissolution rate, so as to improve the body's immunity ability, alleviate tissue damage, and reduce the effect of cohesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of non-ionic surface-active bodies with liquid-phase core cavitation
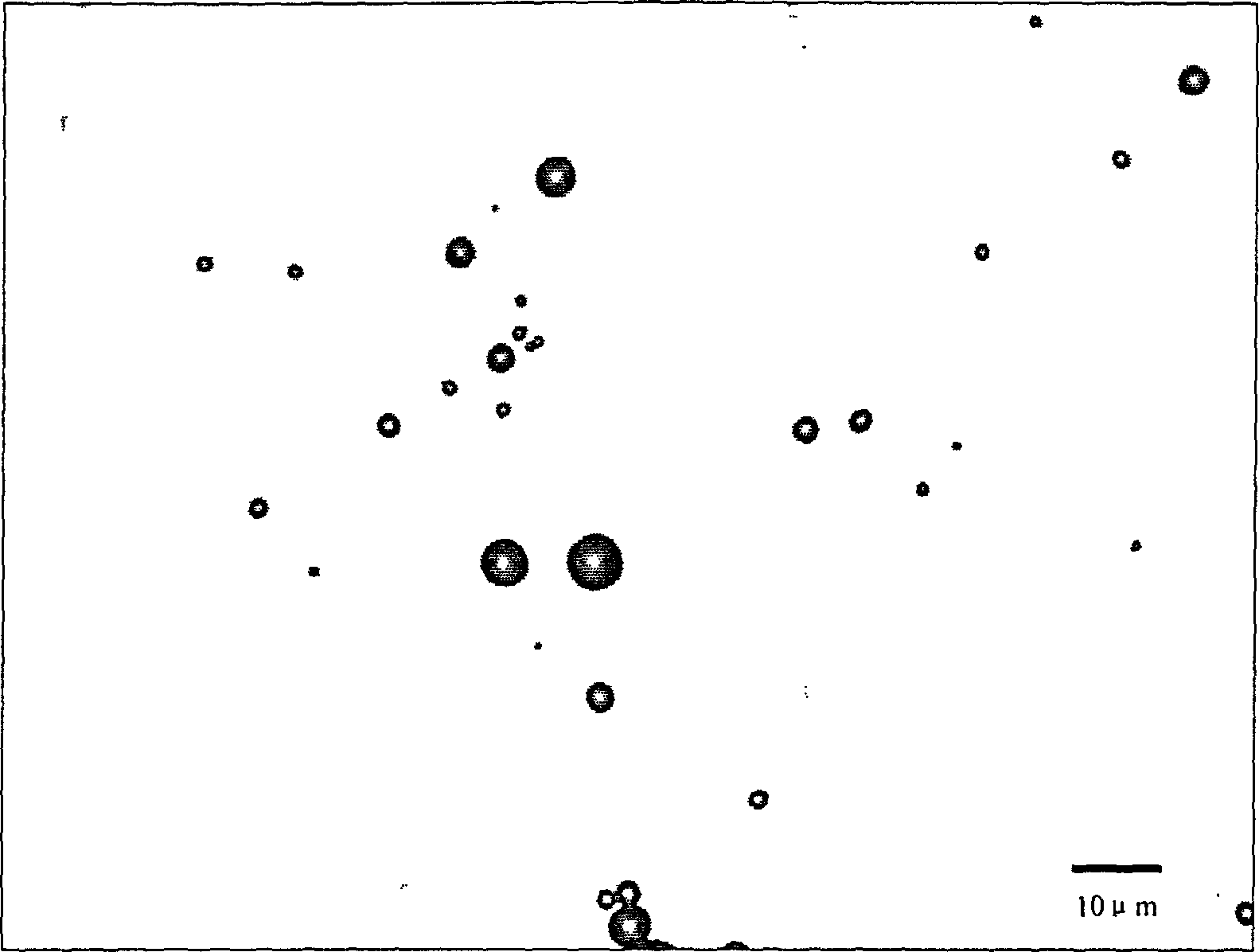
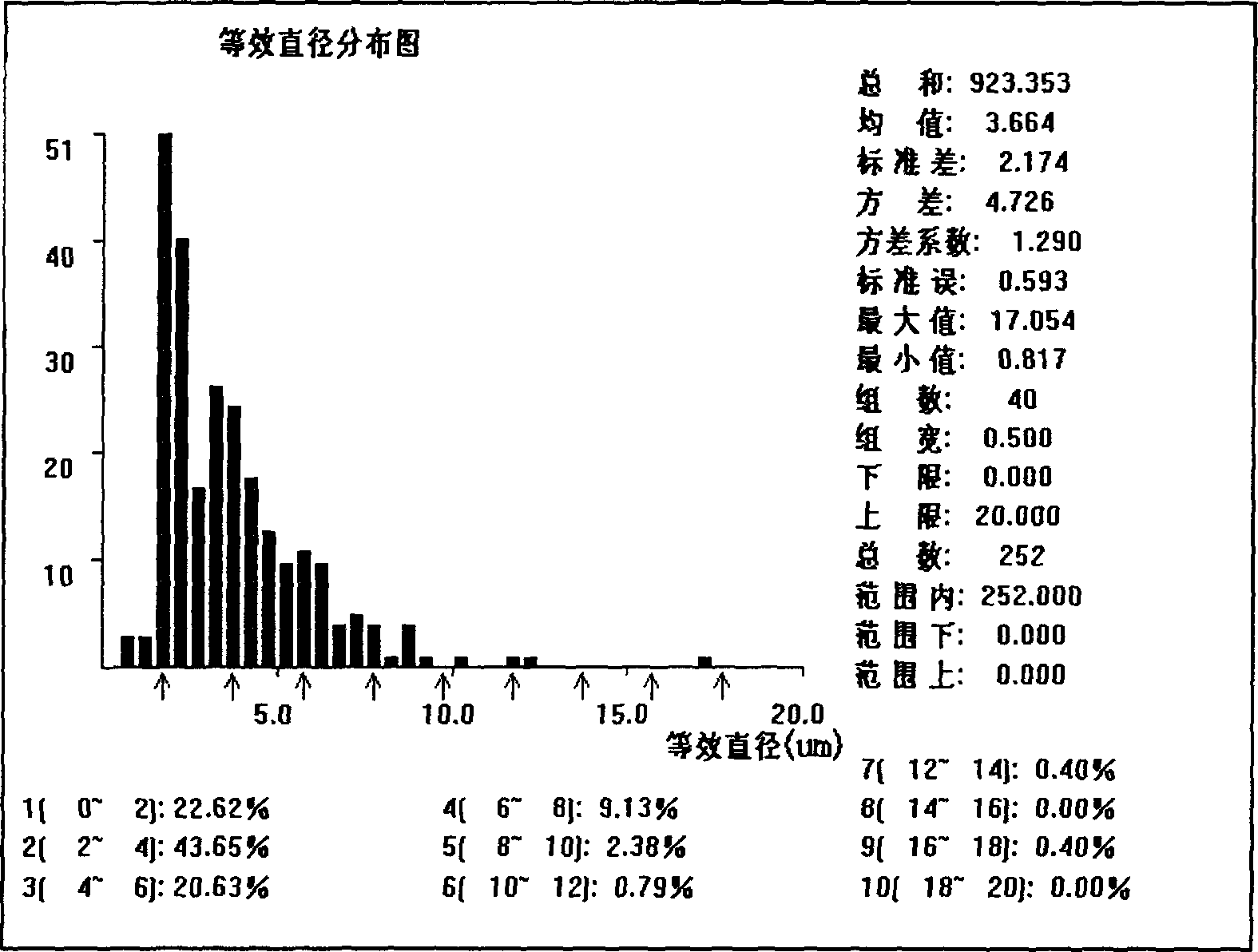
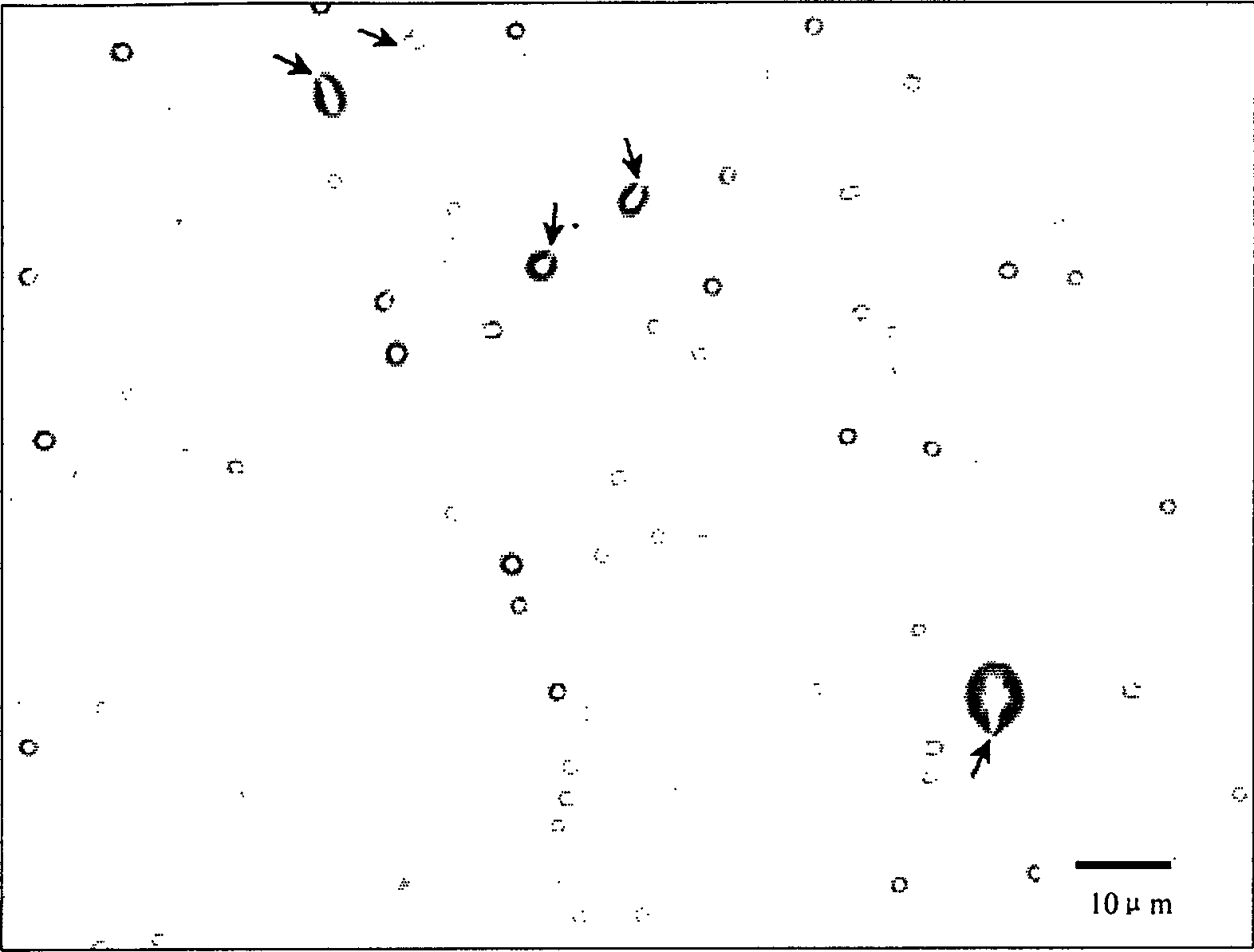
[0042] First, 1 gram of maltodextrin was ground to micron-sized powder particles, 100 mg of Span 60 and 20 mg of cholesterol were mixed in chloroform organic solution, and loaded into a sprayer. Introduce a 50 ml round-bottomed ground flask, spray on the maltodextrin, connect to a rotary evaporator, vacuumize in a 60-degree water bath, and rotate to evaporate for 30 minutes to form a film on the maltodextrin powder particles. The maltodextrin covered by the film was taken out, and air-dried at room temperature for 12 hours. Dissolve the above-mentioned membrane-wrapped maltodextrin in 10 ml of normal saline quickly, shake it manually for 30 seconds, take the suspension and observe it under a microscope. The distribution is about 90%. At room temperature and without sealing, the microvesicles can remain in the flask for 3 days without disappearing or breaking; in the sealed st...
Embodiment 2
[0043] Example 2: Adjusting the HLB value to prepare liquid-phase core cavitation non-ionic surface-active bodies
[0044]Grind 1 gram of maltodextrin to micron-sized powder particles. The HLB values of Span 80 and Tween 80 are calculated according to the weighted average of the respective HLB values to prepare a composite emulsifier with an HLB value of 6, wherein Span 80 (166 mg) accounts for 83% by weight and Tween 80 (34 mg) accounts for 17% by weight , the above compound emulsifier and 20mg of cholesterol were miscible in chloroform organic solution, and loaded into the sprayer. Introduce a 50 ml round-bottomed ground flask, spray on the maltodextrin, connect to a rotary evaporator, vacuumize in a 60-degree water bath, and rotate to evaporate for 30 minutes to form a film on the maltodextrin powder particles. The maltodextrin covered by the film was taken out, and air-dried at room temperature for 12 hours. The above-mentioned membrane-wrapped maltodextrin was quick...
Embodiment 3
[0045] Example 3: Adjusting the HLB value to prepare liquid-phase core perfluoropropane gas cavitation non-ionic surface-active bodies
[0046] Grind 1 gram of maltodextrin to micron-sized powder particles. The HLB values of Span 80 and Tween 80 are calculated according to the weighted average of the respective HLB values to prepare a composite emulsifier with an HLB value of 6, wherein Span 80 (166 mg) accounts for 83% by weight and Tween 80 (34 mg) accounts for 17% by weight , the above compound emulsifier and 20mg of cholesterol were miscible in chloroform organic solution, and loaded into the sprayer. Introduce a 50 ml round-bottomed ground flask, spray on the maltodextrin, connect to a rotary evaporator, vacuumize in a 60-degree water bath, and rotate to evaporate for 30 minutes to form a film on the maltodextrin powder particles. The maltodextrin covered by the film was taken out, and air-dried at room temperature for 12 hours. The above-mentioned film-wrapped malt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com