Anti-oxidizing derivative of aspirin and its synthetic method and use
A technology for aspirin and a synthesis method is applied in the field of antioxidant derivatives of aspirin and the synthesis thereof, and can solve the problems such as failure to completely solve the gastrointestinal injury of NSAIDs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
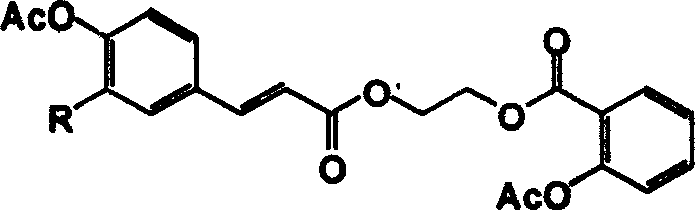
[0010] Example 1: Synthesis of 2-acetoxy-benzoic acid-2-[3-(4-acetoxy-phenyl)-acryloyloxy]-ethyl ester (hereinafter referred to as compound 1)
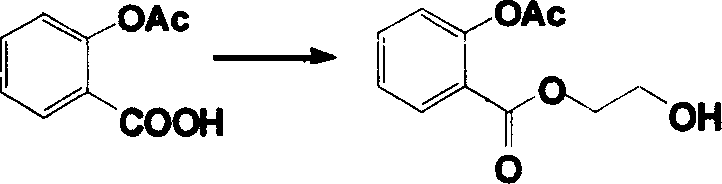
[0011] step one:
[0012]
[0013] Aspirin Compound 3
[0014] Take 5mmol aspirin and 40mmol thionyl chloride to react at 70°C for 2h, then distill off excess thionyl chloride under reduced pressure. The obtained aspirinyl chloride was dissolved in 10 mL of acetone, and added dropwise to the acetone solution in which 20 mmol of ethylene glycol and 0.012 mol of triethylamine were dissolved (0□). 0□Continue to react at room temperature for 1h after reacting for 1h. After the solvent was evaporated, 0.63 g of compound 2-acetoxy-benzoic acid-2-hydroxy-ethyl ester (hereinafter referred to as compound 3) was obtained as yellow oil, with a yield of 56.0%. Physical constants and spectral data of compound 3: R f 0.18 (petroleum ether / ethyl acetate, 1:1). IR(KBr)cm -1 : 3519, 1769, 1723, 1607, 1580, 1485, 1453. 1 H NMR (CDCl 3 ...
Embodiment 2
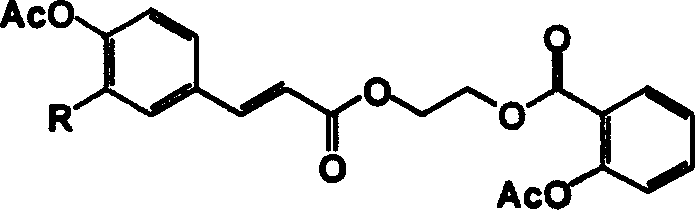
[0023] Example 2: 2-acetoxy-benzoic acid-2-[3-(3-methoxy-4-acetoxy-phenyl)-acryloyloxy]-ethyl ester (hereinafter referred to as compound 2) synthesis
[0024] step one:
[0025]
[0026] Compound 5 Compound 7
[0027] Dissolve 0.08 mol of sodium hydroxide in 30 mL of water, ice-bath to below 10°C, add 0.03 mol of 4-hydroxy-3-methoxyphenylacrylic acid (hereinafter referred to as compound 5) under the protection of argon, and stir until dissolved. Slowly add 0.0375mol acetic anhydride dropwise. After the addition, return to room temperature and react for 1 h, acidify to pH 2-3 with 10% sulfuric acid, filter, wash the filter cake four times with water and recrystallize with ethanol to obtain light yellow solid 4-acetoxy-3-methoxyphenylacrylic acid ( Hereinafter referred to as compound 7) 8.83g, yield 87.6%, R f 0.43 (chloroform:methanol, 6:1). The feeding ratio of the sodium hydroxide to the compound 5 is 2-20:1 (molar ratio). The feed ratio of the acetic anhydrid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com