Method for inducing immune response using HBs DNA vaccine inoculated by HBV core protein reinforcing gene gun
A core protein, hepatitis B virus technology, applied in gene therapy, antiviral agents, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1D
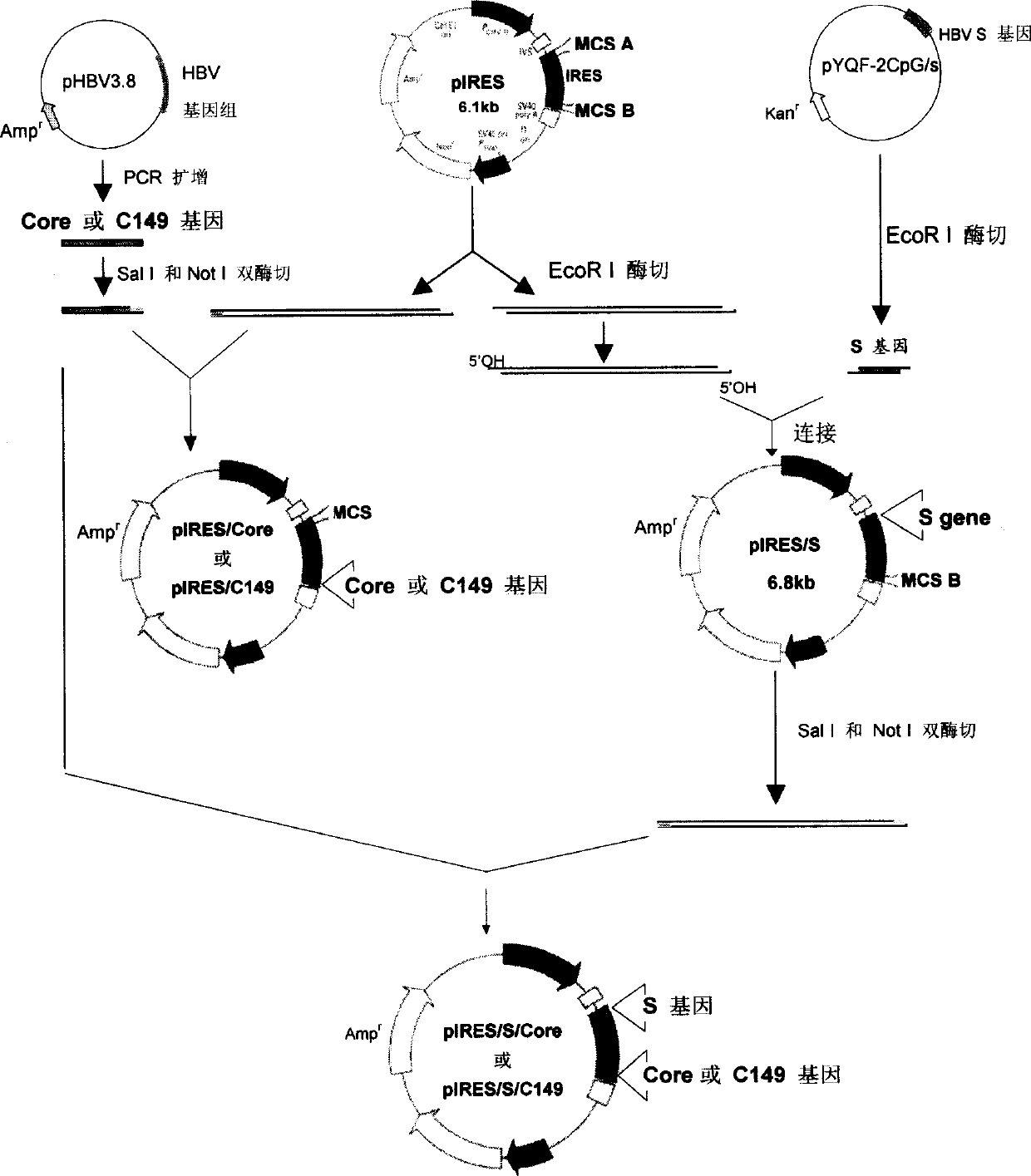
[0046] Expression of Example 1 DNA Vaccine Vector in Mammalian Cells
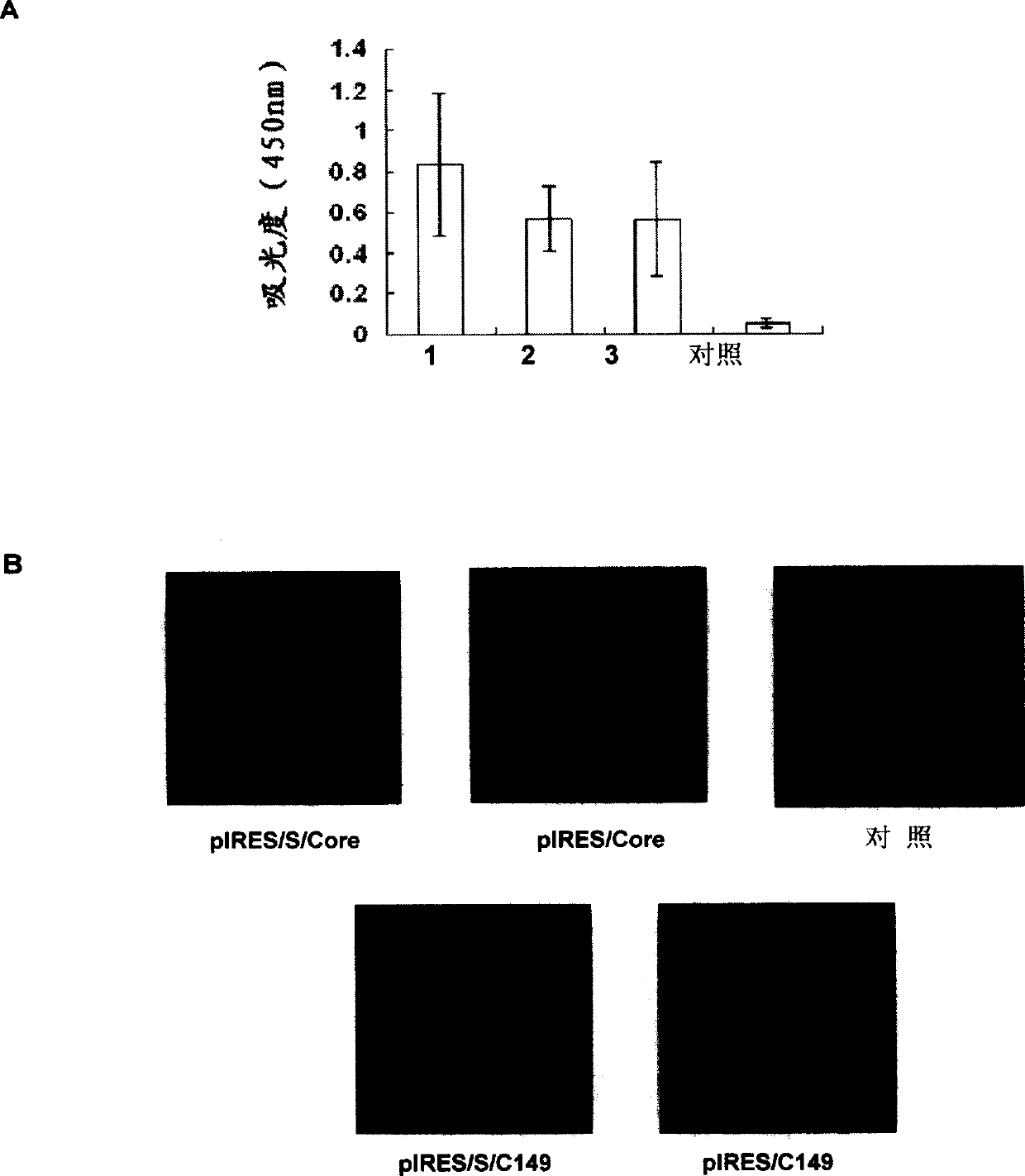
[0047] Plasmids pIRES / S, pIRES / S / Core and pIRES / S / C149 were transiently transfected into HepG2 cells by calcium phosphate method, and the expression of HBsAg in the supernatant of transfected cells was detected by ELISA 48 hours later. The control was the supernatant of untransfected cells. The results are shown in Figure 2A, pIRES / S, pIRES / S / Core and pIRES / S / C149 can all express HBsAg, the OD450nm of each group is 0.832±0.349, 0.57±0.162 and 0.561±0.277, and the control is 0.052±0.024.
[0048] The plasmids IRES / Core, IRES / S / Core, IRES / C149, and IRES / S / C149 were transiently transfected into Cos7 cells by liposome method, and 48 hours later, anti-HBV Core monoclonal antibody and Rhodanmin-labeled goat anti-mouse secondary antibody were used For immunofluorescence staining. Laser confocal scanning results showed that Core protein and its truncated C149 protein were stained with red fluorescence by Rhodanmi...
Embodiment 2
[0049] HBcAg-specific humoral immunity level after embodiment 2 immunization
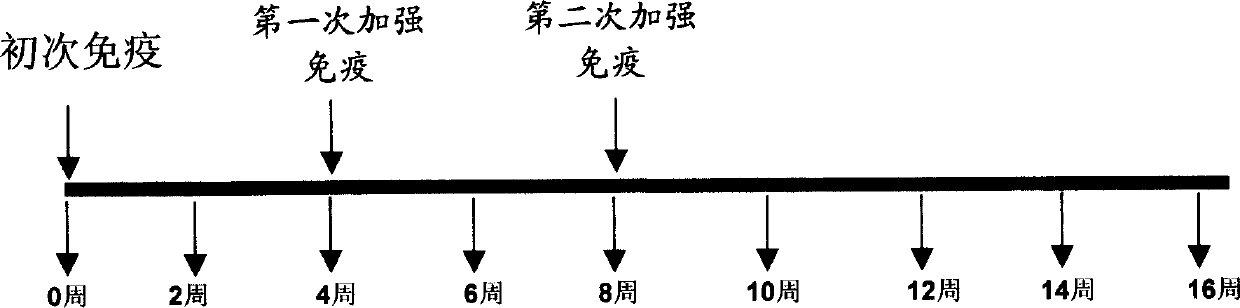
[0050] BALB / c mice were randomly divided into 8 groups, 6 mice in each group. The first booster was given in the 4th week after the initial immunization, and the second booster was given in the 8th week. Two weeks after the second booster (10 Week) HBcAg-specific IgG level and IgG subclass level in serum. The control was untreated mouse serum. The results are shown in Figure 3. All groups inoculated with plasmids expressing full-length HBcAg or HBcAg1-149 truncated forms could induce HBcAg-specific IgG antibodies, and there was no statistical difference among the groups. Further analysis of the subclasses in each group found that the anti-HBcAg subclasses of pIRES / Core, pIRES / S / Core and pIRES / Core+pIRES / S expressing full-length HBcAg were mainly IgG2a, and their IgG2a / IgG1 ratios were 2.47 and 2.73 respectively. and 2.07, while the anti-HBcAg subclasses of pIRES / C149+pIRES / S and pIRES / S / C149 ex...
Embodiment 3
[0051] HBsAg-specific humoral immunity level after embodiment 3 immunization
[0052] In order to monitor the change of anti-HBsAg total IgG titer, the gene gun was inoculated with pIRES / S, pIRES / S / Core, pIRES / Core+pIRES / S, pIRES / S / C149, pIRES / C149+pIRES / S, intramuscular injection Serum collected from pIRES / S and untreated control groups were mixed in equal volumes in each group, and the titer level was detected by ELISA method. The results are shown in Figure 5A. At the 14th week, the antibody titers in each group were 1:4800, 1:4800, 1:4800, 1:4800 and 1:6400, respectively. The serum levels of HBsAg-specific IgG were detected two weeks after the second booster (week 10) in each group, and the results are shown in FIG. 5B . In addition to the pIRES / Core group and the control group, pIRES / S, pIRES / S / Core, pIRES / Core+pIRES / S, pIRES / C149+pIRES / S, pIRES / S / C149 and intramuscular injection of pIRES / S group can induce HBsAg-specific IgG antibody, there is no statistical differen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com