Super antigen fusion protein for cancer therapy and its producing method
A fusion protein and superantigen technology, applied in the fields of peptide/protein components, animal/human proteins, chemical instruments and methods, etc., can solve the problems of antibody drug cycle and huge investment cost, save drug development costs and reduce medical costs. , the effect of reducing the dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1. Isolation of superantigen SEA gene
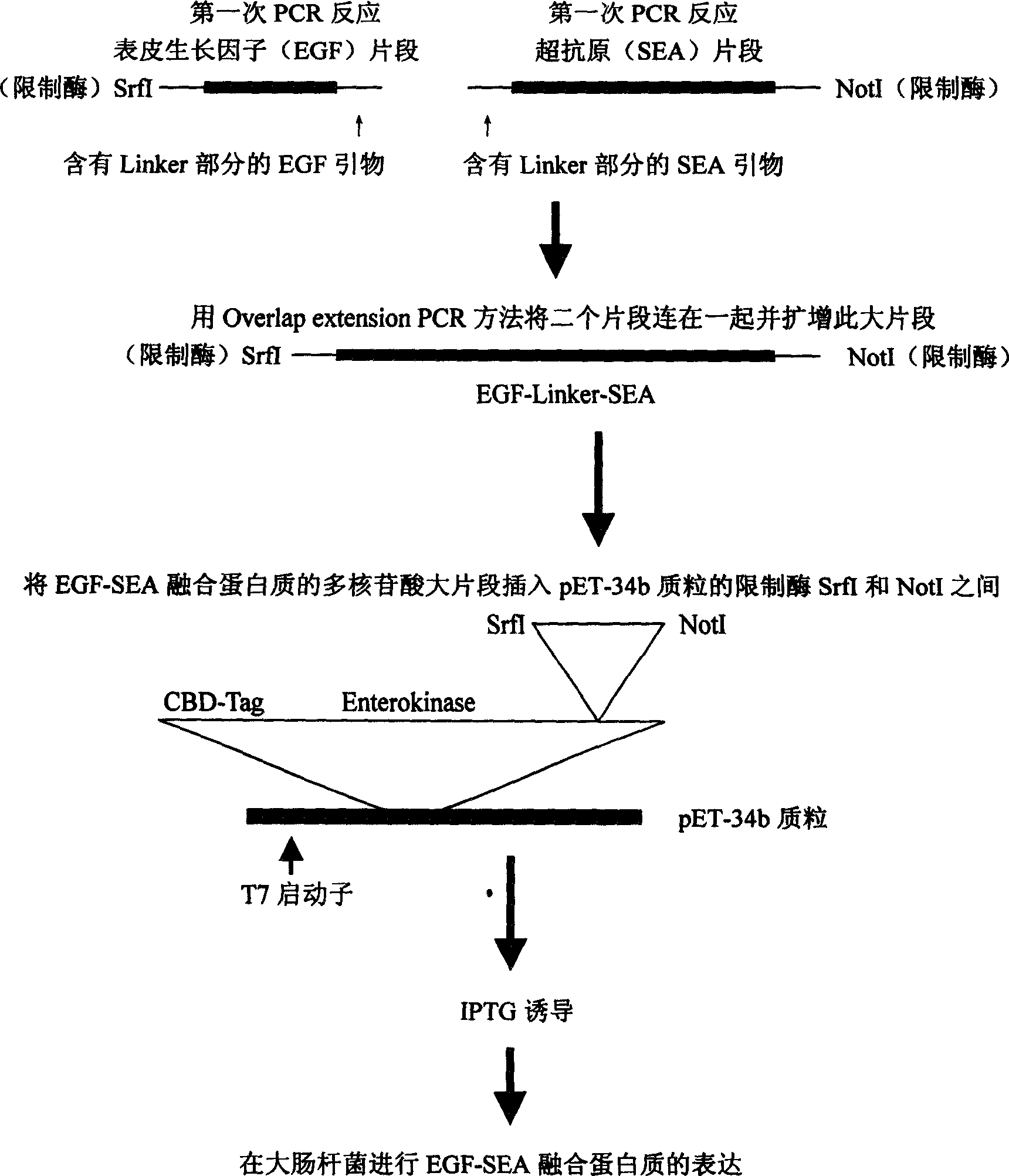
[0048] According to the conventional molecular biology experiment method (T. Maniatis, et al, Molecular cloning, Alaboratory manual, Second edition, Cold spring harbor laboratory, 1989), the phenol / chloroform extraction method was used to extract Staphylococcus aureus (Staphylococcus aureus FRI337) According to the sequence of the superantigen SEA gene in the literature (MJ Betley and JJ Mekalanos, J. Bacteriol., 170, 34-41, 1988), the primers were designed: (1) the forward primer containing the restriction enzyme cut site of SrfI, 5'-GAGCCCGGGCAGCGAGAAAAGCGAAGAAATAAAT-3'; (2) Reverse primer containing NotI restriction site, 5'-GTGCGGCCGCACTTGTATATAAATATATATCAATATGCAT-3'. Use this primer to carry out the PCR amplification reaction of the SEA gene. The amount of template is 0.1 μl, and the cycle conditions of the PCR reaction are: 95°C for 30 seconds → 55°C for 30 seconds → 72°C for 120 seconds, a total of 30 cycles of reaction...
Embodiment 2
[0050] Example 2. Isolation of epidermal growth factor EGF gene
[0051] Design primers based on the reported epidermal growth factor EGF gene sequence (J. Smith, et al, Nucleic Acids Res., 10, 4467-4482, 1982): (1) Forward primer containing SrfI restriction enzyme cleavage site, 5'- GAGCCCGGGCAATTCCGATAGCGAGTGT-3'; (2) Reverse primer containing NotI restriction site, 5'-GTGCGGCCGCTCTAAGTTCCCACCATTT-3'. The EGF gene was isolated from the Human breast carcinoma cDNA gene library (Clontech) by PCR, which encodes a 53 amino acid polypeptide. The amount of template is 0.1 μl, and the cycle conditions of the PCR reaction are: 95°C for 30 seconds → 55°C for 30 seconds → 72°C for 30 seconds, a total of 30 cycles of reactions, and finally 72°C for 10 minutes. The length of the DNA fragment is approximately 170 bp.
[0052] The DNA product was subjected to low melting point gel electrophoresis, the DNA fragment was recovered, and the fragment was treated with SrfI and NotI restriction enzy...
Embodiment 3
[0053] Example 3. Isolation of vascular endothelial cell growth factor VEGF
[0054] From the reported vascular endothelial cell growth factor VEGF gene sequence (PJKeck, et al, Science, 246, 1309-1312, 1989; E. Tischer, et al, J. Biol. Chem., 266, 11947-11954, 1991) The primers for VEGF-121 were designed in: (1) forward primer containing SrfI restriction enzyme cut site, 5'-GAGCCCGGGCGCACCCATGGCAGAAGGAGGA-3'; (2) reverse primer containing NotI restriction enzyme cut site, 5'-GTGCGGCCGCCCGCCTCGGCTTGTCACATTTTTCTTGTCTTGCTCTATCTTTCTT-3 '. The VEGF-121 gene was isolated from the Human breast carcinoma cDNA library (Clontech) by PCR, which encodes a 121 amino acid polypeptide. The amount of template is 0.1 μl, and the cycle conditions of the PCR reaction are: 95°C for 30 seconds → 55°C for 30 seconds → 72°C for 50 seconds, a total of 30 cycles of reactions, and finally 72°C for 10 minutes. The length of the DNA fragment is approximately 370 bp.
[0055] The DNA product was subjected to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com