Dispersible tablet of doxifluridine
A technology for doxifluridine and dispersible tablets, which is applied in the fields of pill delivery, organic active ingredients, and medical preparations containing active ingredients, etc., which can solve the problems of difficult development and achieve more absorption points, faster absorption, and improved drug use. compliance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
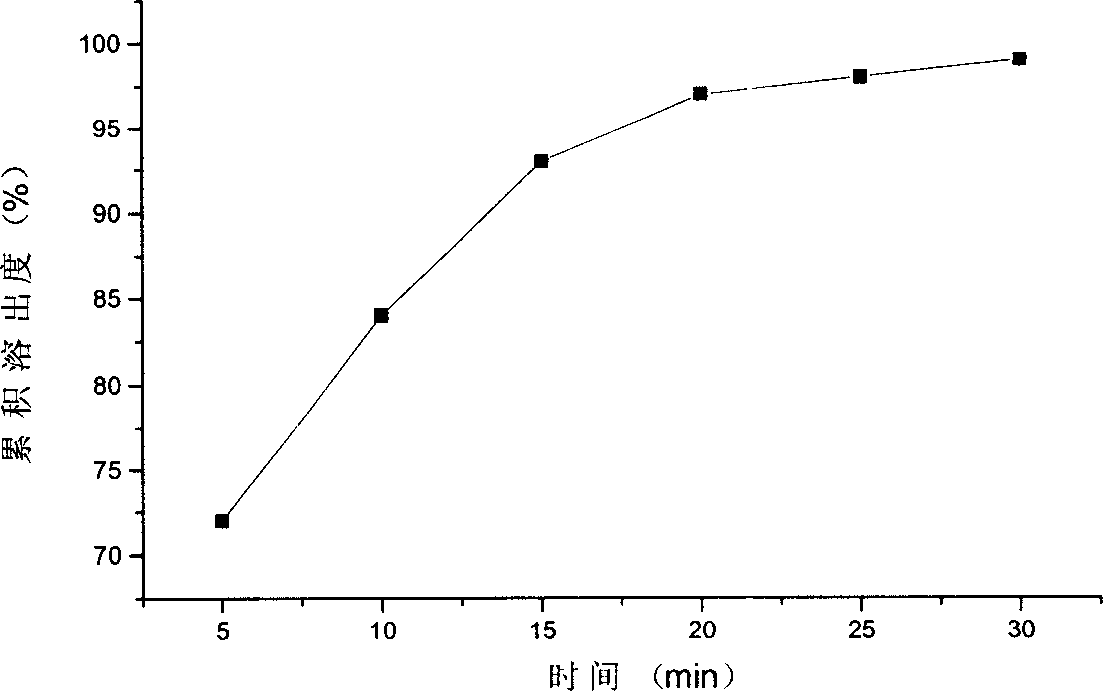
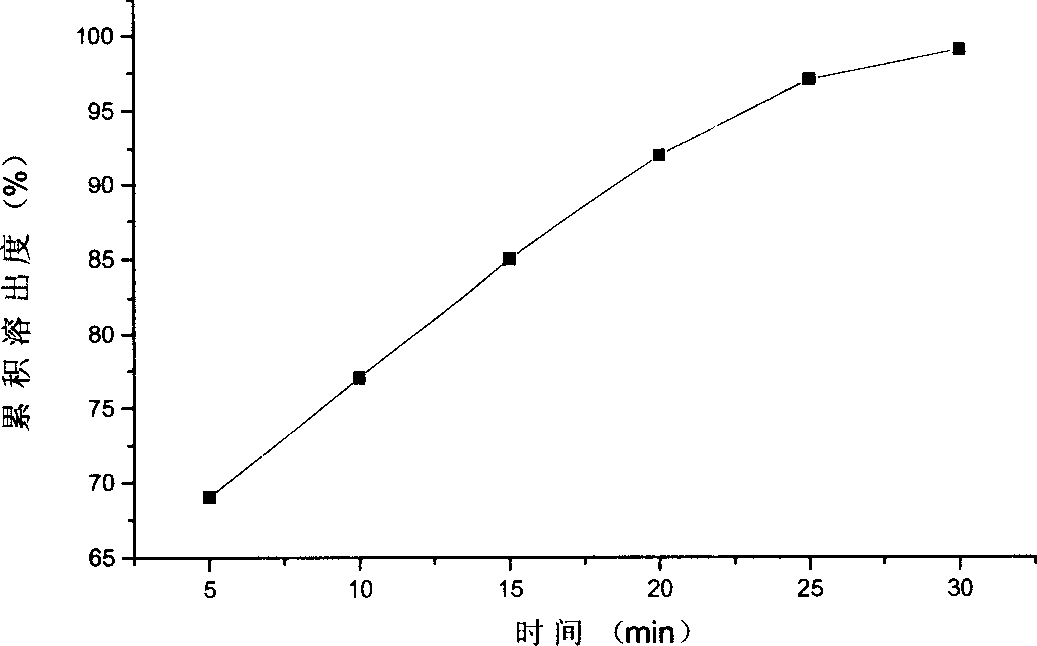
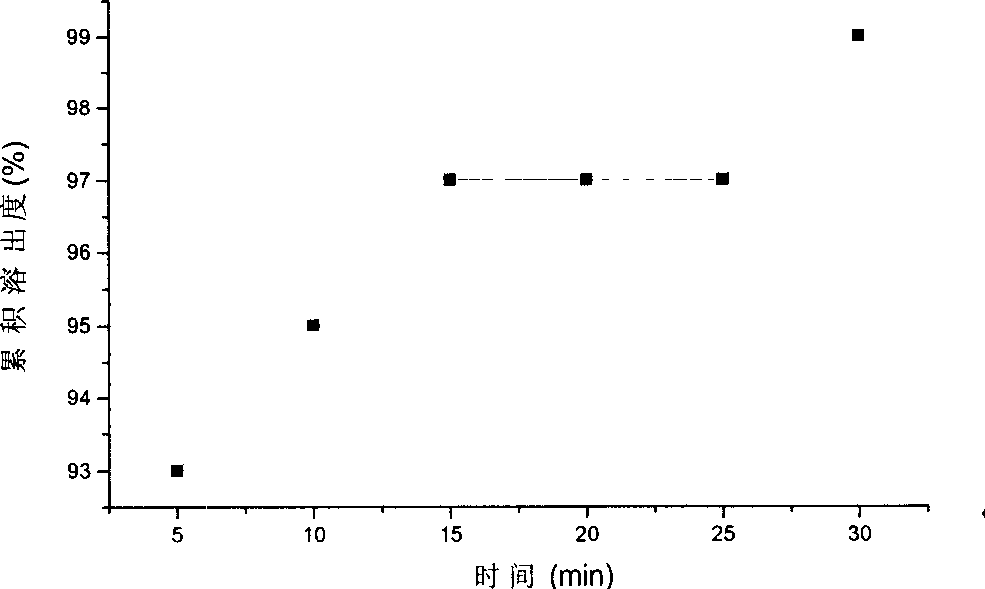
Image
Examples
Embodiment
[0014] Embodiment (prescription quantity is 1000):
[0015] Eight specific prescriptions:
[0016] Prescription A:
[0017] Doxifluridine 20g
[0018] Hydroxypropylcellulose (disintegrant) 96g
[0019] Magnesium stearate (lubricant) 1.5g
[0020] Micronized silica gel (flow aid) 2.5g
[0021] Preparation process: the main drug is passed through a 100-mesh sieve, the disintegrant is passed through a 80-mesh sieve, the prescribed amount of the main drug and the disintegrant are weighed and mixed evenly, the prescribed amount of lubricant is added, mixed evenly, and tabletted to obtain the product. Dispersible tablet dispersion time limit is 1.0min.
[0022] Prescription B:
[0023] Doxifluridine 60g
[0024] Hydroxypropyl cellulose (disintegrant) 108g
[0025] 10% starch slurry (adhesive) 100g
[0026] Magnesium stearate (lubricant) 2g
[0027] Preparation process: The main drug is passed through a 100-mesh sieve, and the disintegrant is passed through a 80-mesh sieve....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com