Industrial preparation method for 3-amino phenylacetic acid
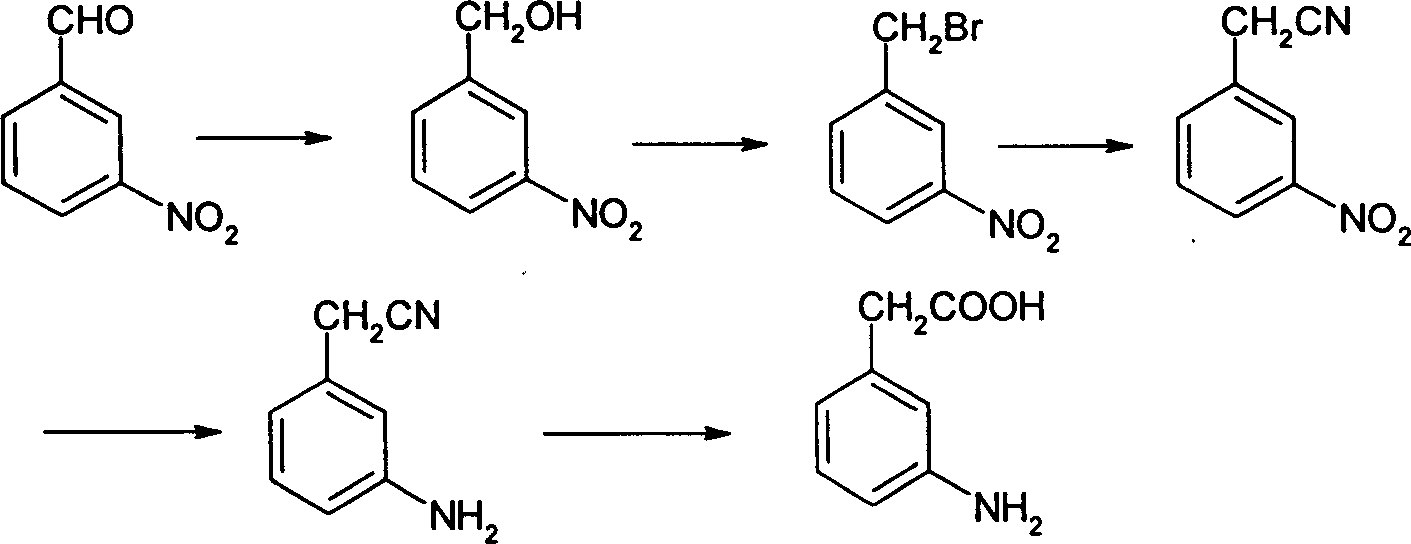
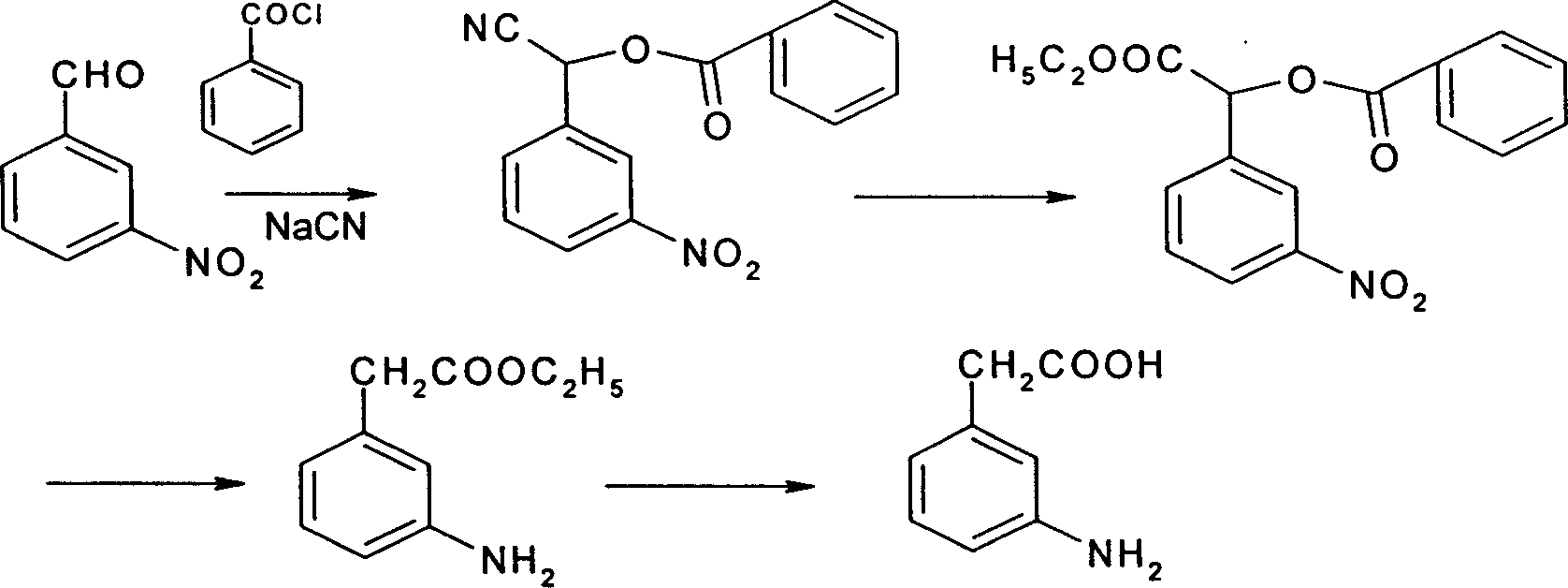
A technology of aminophenylacetic acid and p-nitrophenylacetonitrile, which is applied in the field of preparation of 3-aminophenylacetic acid, can solve the problems of nitration products being very complex and difficult to separate, and achieves the effects of high selectivity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
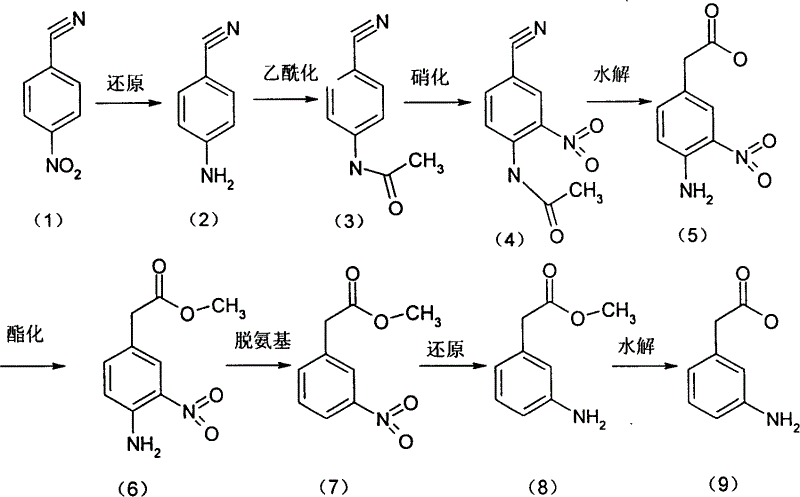
[0028] Add 200ml of water into the three-necked flask, add 80g of iron powder under mechanical stirring, reflux with 4ml of acetic acid for 30min, cool down to 90-95°C, add 64.8g of p-nitrophenylacetonitrile in batches, reflux for 4hr after the addition, and cool down to 80°C , add 200ml of dichloroethane, filter with suction, wash the filter cake with hot dichloroethane, separate the organic layer, and precipitate under reduced pressure to obtain 50.1 g of the product p-aminophenylacetonitrile, with a yield of 95%.
[0029] Add 24.5ml acetic acid to 52.8g p-aminophenylacetonitrile, add dropwise 24.5ml acetic anhydride at room temperature, continue to react for 1hr after dripping, the reaction solution is poured into ice water, and suction filtration obtains 63.3g of product p-acetamidophenylacetonitrile, the yield 91%.
[0030] Add 91ml of 60% nitric acid dropwise to 91ml of concentrated sulfuric acid, add 34.8g of p-acetaminophenylacetonitrile in batches under ice water cool...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com