Process for producing propylene and hexene from C4 olefin streams
A technology of logistics and hexene, applied in chemical instruments and methods, organic chemical methods, hydrocarbon cracking to produce hydrocarbons, etc., can solve problems such as increasing the cost of towers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 125
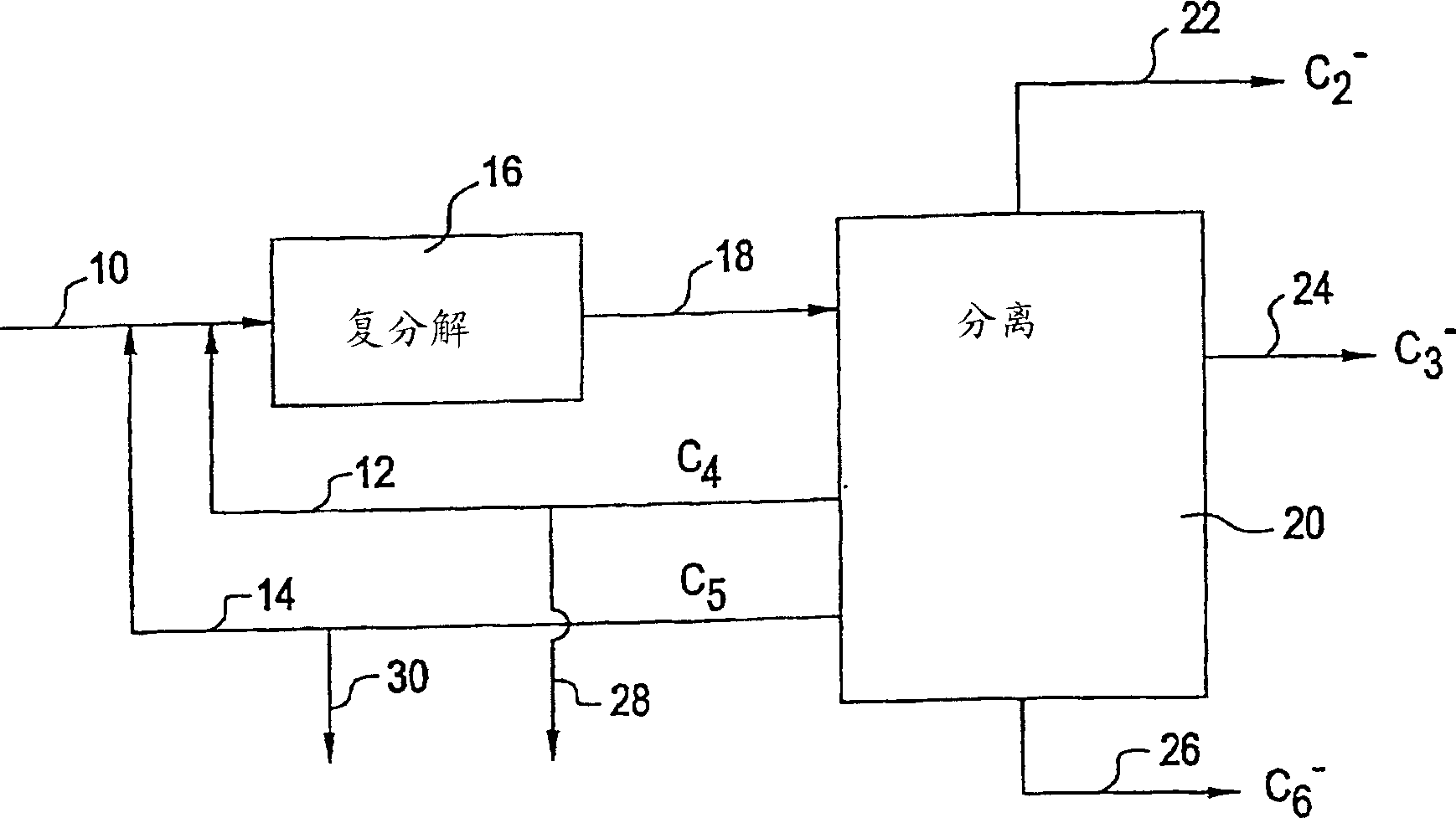
[0076] Example 125 is a feed with a high content of 2-butene in the feed. When there is no pentene recycling, the reaction conversion rate is 35%, and propylene and pentene are produced almost equimolarly by the following reactions:
[0077]
example 148
[0078] Example 148 uses 2-pentene to replace part of 2-butene to simulate component recycling. It can be found that the total pentene produced is reduced by 34% (44 to 29), propylene is increased by 3%, and hexene is increased by 3 times. Obviously, the butene conversion rate has also increased from 35 to 48%, resulting in greater yields of propylene and hexene.
example 119
[0079] Example 119 represents the C of a substantially pure 1-butene feed 4Feed. Under the same operating conditions, the conversion rate is about 45%. However, the selectivity of 7% for propylene and 8% for pentene is low, indicating that the concentration of 1-butene in the feed is high and the lack of any specific isomerization catalyst mixed with the metathesis catalyst. In Example 150, 2-pentene was used instead of some 1-butene. The amount of 2-pentene was slightly less than the 2-pentene produced in the Example 119 reaction. The conversion rate is still basically the same. The pentene cycle has two functions. The increased selectivity of propylene and hexene indicates that 2-pentene reacts with 1-butene. Second, the presence of 2-pentene in the feed inhibits the equilibrium reaction of 1-butene and 2-butene (formed by the self-isomerization of 1-butene on the metathesis catalyst). Therefore, generally, pentene is not formed as the final reaction product. Optionally, it will...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap