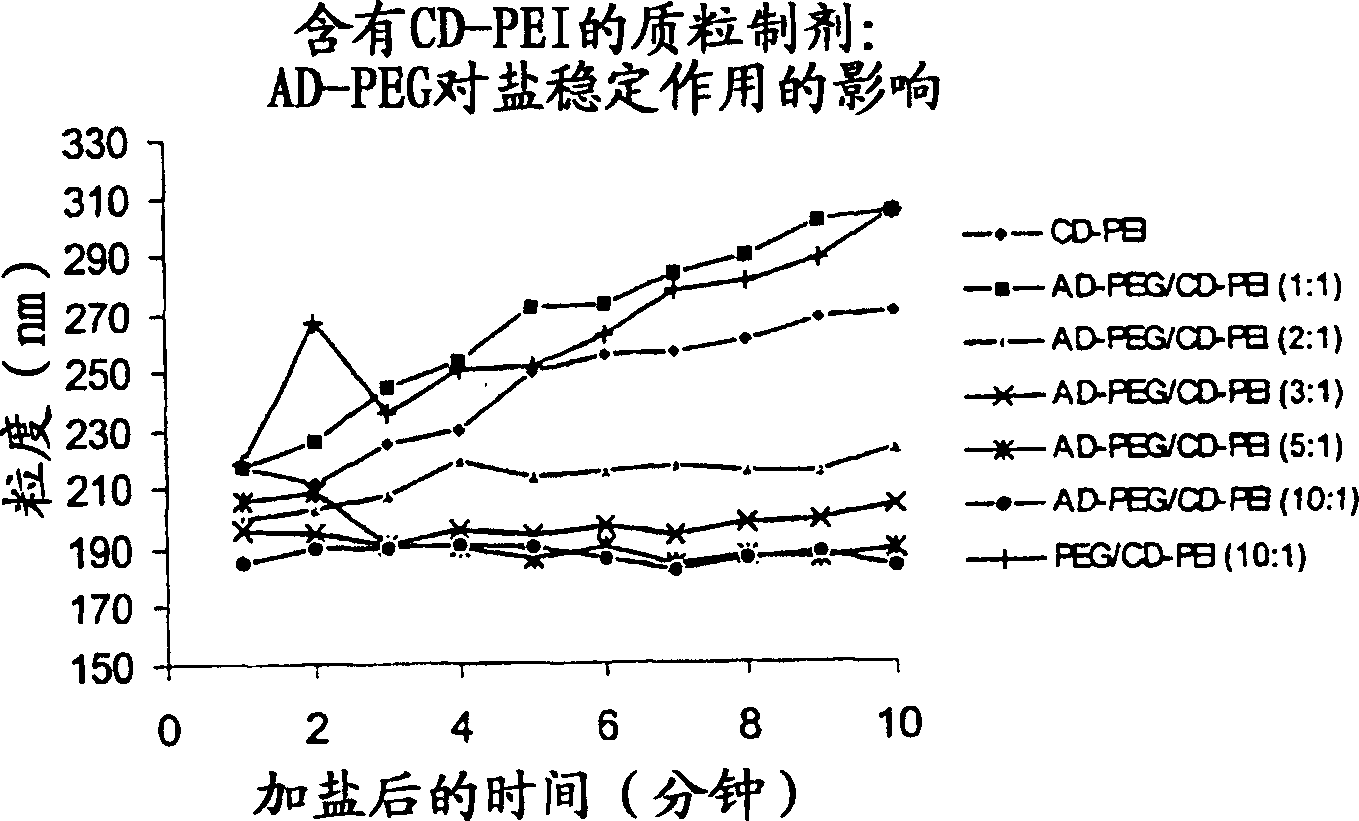
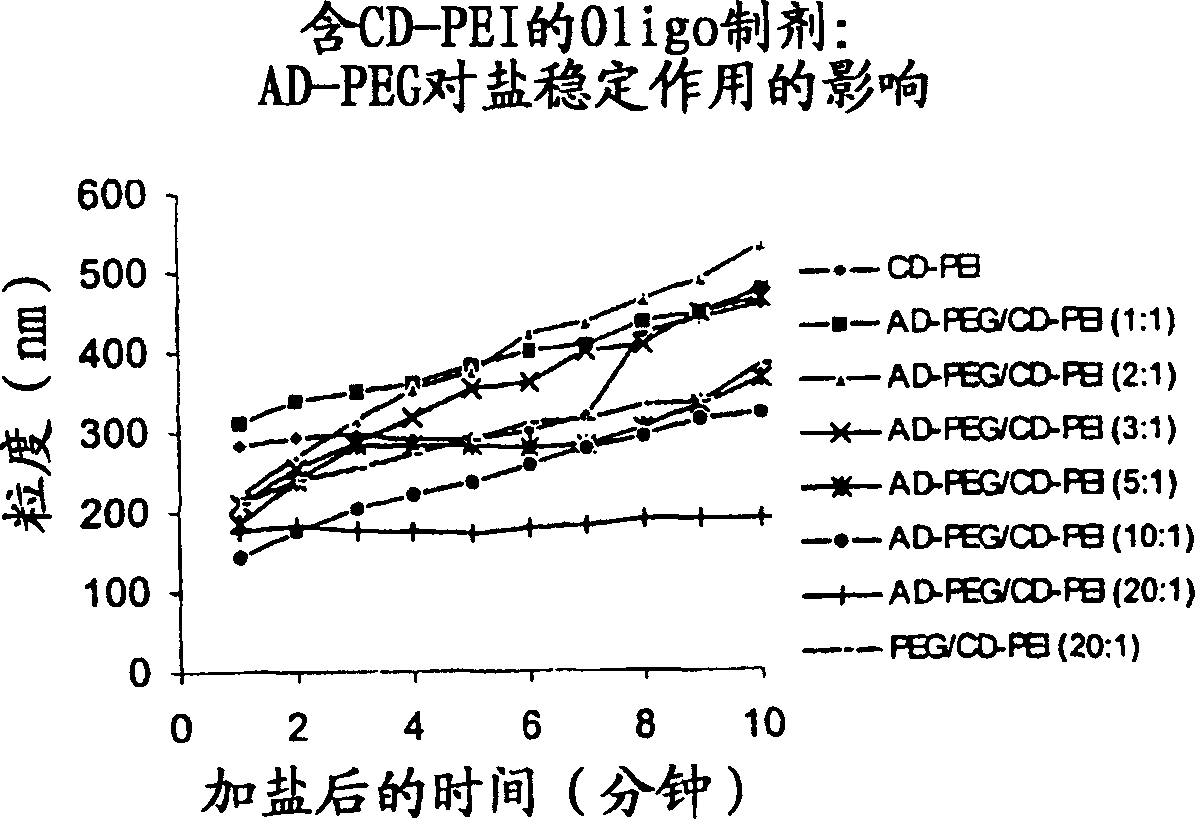
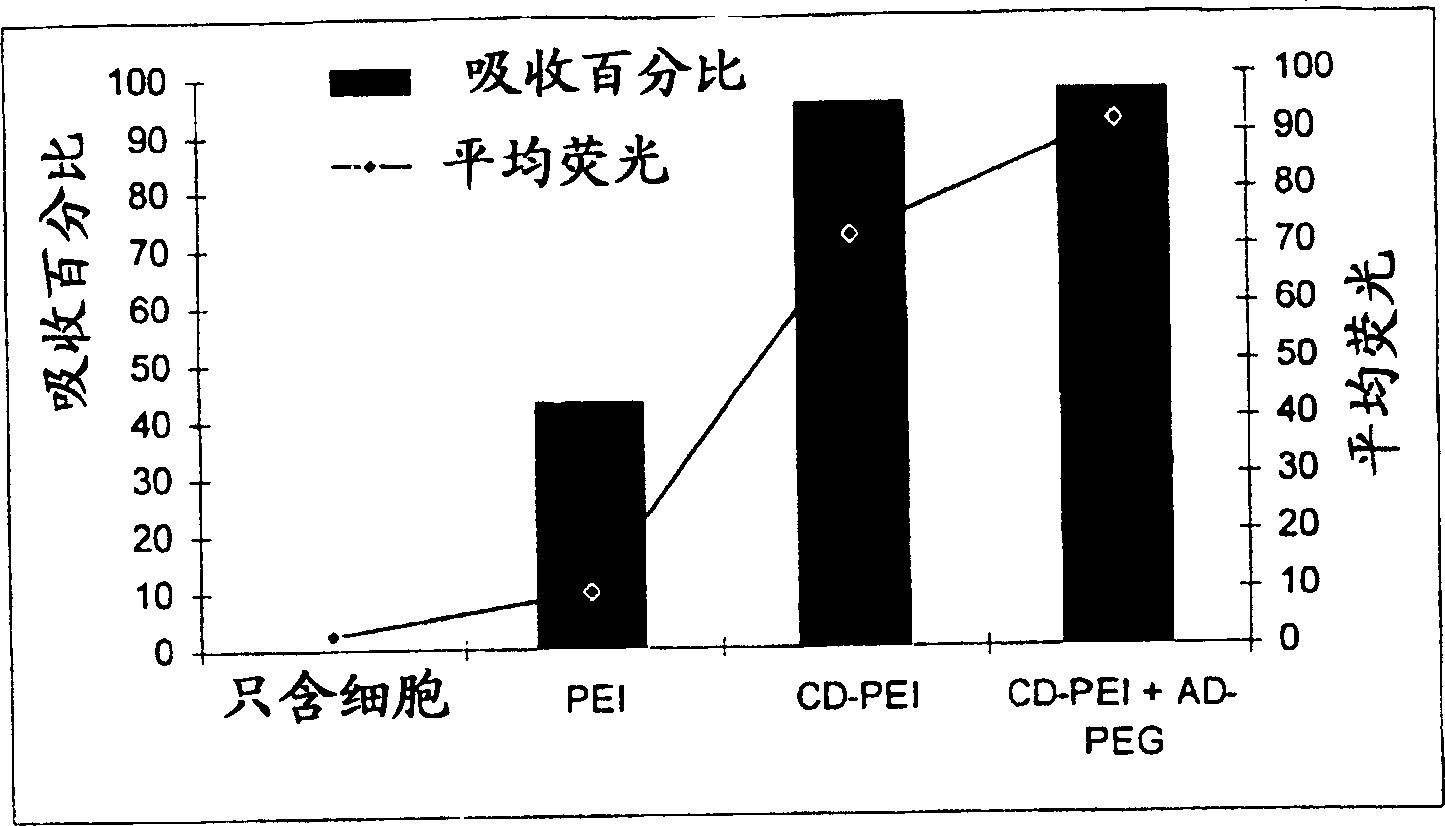
Carbohydrate-modified polymers, compositions and uses related thereto
A carbohydrate and polymer technology, applied in the field of wheat, fucose, sucrose, newly discovered, modified, and lactose, can solve problems such as difficult control of pretreatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Example 1 Synthesis and Characterization of CD-bPEI Loaded with Different CDs
[0136]
[0137] put branch PEI 25,000 (295.6mg, Aldrich) and 6-monotosyl-β-cyclodextrin (2.287g, Cyclodextrin Technologies Development, Inc.) were dissolved in 100mL different H 2 O / DMSO solvent mixture (Table 1). The resulting mixture was stirred at 70°C for 72 hours. The solution gradually turned yellow. The solution was then transferred to a Spectra / Por MWCO 10,000 membrane and dialyzed against water for 6 days. It was then lyophilized to remove water to obtain a light-colored solid. based on 1 H NMR (Varian 300MHz, D 2 O) δ5.08ppm (s br., C of CD 1 H), 3.3-4.1ppm (m br. CD of C 2 H-C 6 H), 2.5-3.2ppm (CH of m br. PEI 2 ) to calculate the cyclodextrin / PEI ratio.
[0138] It was found that the cyclodextrin loaded on PEI increases with the H in the reaction mixture 2 The amount of O decreased and increased (Table 1).
[0139] h 2 O / DMSO(mL)
Embodiment 2
[0140] The synthesis of embodiment 2 linear PEI-CD
[0141]
[0142] Low loading: Linear PEI (50 mg, Polysciences, Inc., MW 25,000) was dissolved in anhydrous DMSO (5 mL). To the solution was added cyclodextrin monotosylate (189 mg, 75 equivalents, Cyclodextrin Technologies Development, Inc.). The solution was stirred at 70-72°C for 4 days under argon atmosphere. The solution was then dialyzed against water (total dialyzed volume approximately 50 mL) for 6 days (Spectra / Por7 MWCO 25,000 membrane). lPEI-CD (46 mg) was obtained after lyophilization. 1 H NMR (Bruker AMX500MHz, D 2 O) δ5.09 (s br., C of CD 1 ), 3.58-4.00 (m br. CD of C 2 -C 6 ), 2.98 (m br. PEI). 8.8% of PEI repeat units were attached to CD.
[0143] High loading: Linear PEI (50 mg, Polysciences, Inc., MW 25,000) was dissolved in anhydrous DMSO (10 mL). To the solution was added cyclodextrin monotosylate (773 mg, 300 equivalents, Cyclodextrin Technologies Development, Inc.). The solution was stirred...
Embodiment 3
[0144] Synthesis and Characterization of CD-1PEI Loaded with Different CDs in Example 3
[0145]
[0146] Linear PEI 25,000 (500 mg, Polysciences, Inc.) and 6-monotosyl-β-cyclodextrin (3.868 g, Cyclodextrin Technologies Development, Inc.) were dissolved in 36 mL of DMSO. The resulting mixture was stirred at 70°C for 6 days. The solution gradually turned yellow. The solution was then transferred to a Spectra / Por MWCO 10,000 membrane and dialyzed against water for 6 days. It was then lyophilized to remove water to obtain a light-colored solid. based on 1 H NMR (Varian 300MHz, D 2 O) δ5.08ppm (s br., C of CD 1 H), 3.3-4.1ppm (m br. CD of C 2 H-C 6 H), 2.5-3.2ppm (CH of m br. PEI 2 ) to calculate the cyclodextrin / PEI ratio. In this example, the cyclodextrin / PEI ratio was 8.4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com