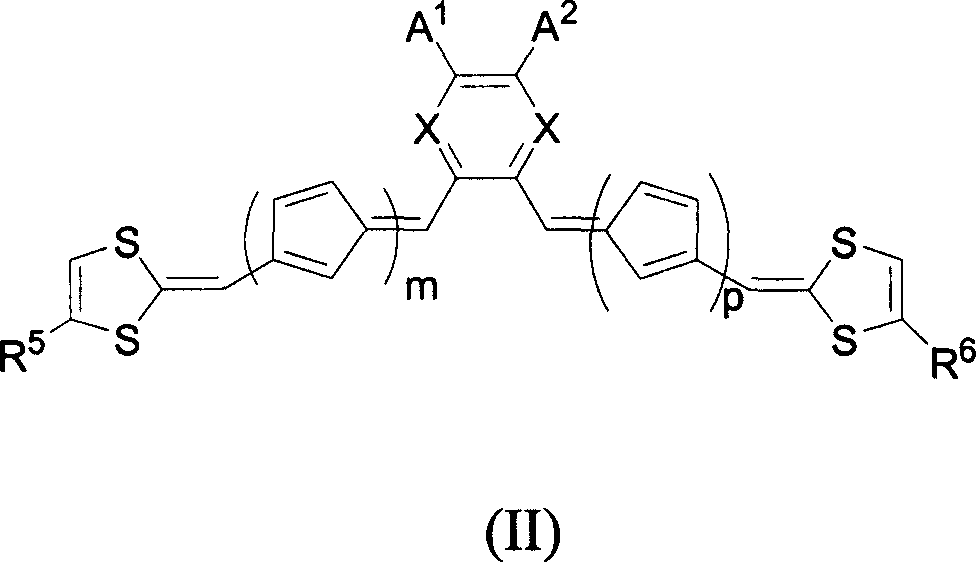
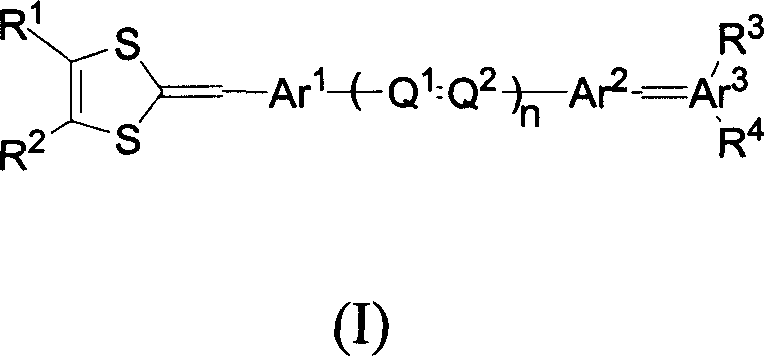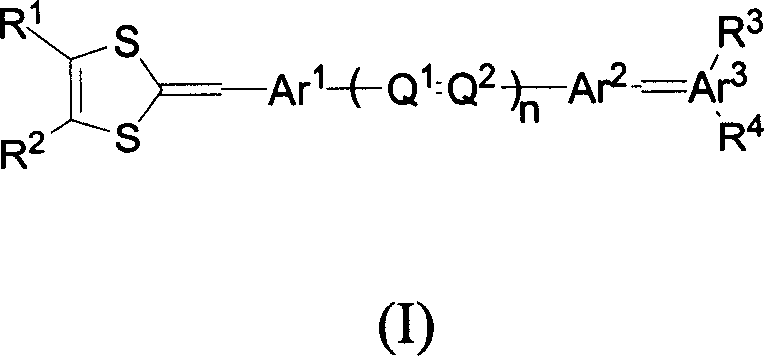Organic non-linear optical chromophore compound containing 1,3-dithio cyclopentenyl group
A technology based on dithiocyclopentenyl and nonlinear light, which is applied to the application field of the above-mentioned compounds in polarized polymers, and can solve the problems of decreased polarization efficiency, smaller average orientation factor of chromophore molecules, and reduced color development. Problems such as the ground state dipole moment of cluster molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of Organic Chromophore Compound 4-(2,5,7,9-tetrathiobicyclo[4.3.0]-nonenylene)-4'-(fluorenylene)styrenebenzene (1)
[0037]
[0038] 1) 2,5,7,9-tetrathiobicyclo[4.3.0]-nonene-1(6)-8-triphenylphosphonium salt (2) Synthesis with reference to literature (A.J.Moore, M.R.Bryce, synthesis, 26 -28, 1990). MS (TOF): 454 (M-1).
[0039]
[0040] 2) Synthesis of p-fluorenylidene toluene (3):
[0041]
[0042] in N 2 Under atmosphere, add 30mL of freshly steamed absolute ethanol to a 100mL three-necked round-bottomed flask containing an electromagnetic stirrer, and then add 1.00g of Na metal cut into small pieces. After the reaction of Na was completed, 3.32 g of fluorene was added thereto under stirring. Heat to 50°C, then add 3.00g p-tolualdehyde / 10mL absolute ethanol solution. Reaction at this temperature for 6h, cooled to room temperature. The solid was collected by filtration to obtain 3.46 g of a yellow solid, and the product was pure enough to direc...
Embodiment 2
[0052] Synthesis of Organic Chromophore Compound 2-(2,5,7,9-tetrathiobicyclo[4.3.0]-nonenylene)-5-[p-fluorenylenestyryl]thiophene (6)
[0053]
[0054] 1) Synthesis of 2-(2,5,7,9-tetrathiobicyclo[4.3.0]-nonenylidene-1(6)-8-yl)-5-thiophenecarbaldehyde (7):
[0055]
[0056] in N 2 Under protection, 1.60 g of the phosphonium salt 2 synthesized in Example 1 above, 0.14 g of thiophene dicarbaldehyde were added to a 100 mL three-neck round bottom flask, and then 30 mL of freshly steamed tetrahydrofuran and excess triethylamine were added. The reaction was stirred at room temperature for 16 h, the solvent was spin-dried, and the obtained substance was dissolved in dichloromethane, washed with water and saturated brine respectively, and anhydrous MgSO 4 After drying overnight, remove the desiccant, spin dry and separate by column chromatography. 0.266 g of a yellow solid substance was obtained. MS (EI): 316 (M + ), 288, 168.
[0057] 2) Synthesis of compound 6:
[0058] i...
Embodiment 3
[0060] Synthesis of Organic Chromophore Compound 4-(2,5,7,9-tetrathiobicyclo[4.3.0]-nonenylene)-4'-(fluorenylidene)benzylidene (8)
[0061]
[0062] 1) Synthesis of 4-fluorenylidene-4'-nitrobenzene (9)
[0063]
[0064] in N 2 Under the atmosphere, add 20 mL of freshly steamed absolute ethanol to a 100 mL three-neck round-bottomed flask containing an electromagnetic stirrer, and then add 0.28 g of Na metal cut into small pieces. After the Na reaction is complete, add 1.00g fluorene to it under stirring, heat to 50°C, then add 0.91g p-nitrobenzaldehyde / 20mL absolute ethanol solution, react at 50°C for 6h, cool, and filter to obtain yellow slightly Red solid substance 0.46g, MS (HR-EI): 299.0952 (M + ) (Cal. 299.0946).
[0065] 2) Synthesis of p-fluorenylidene aniline (10)
[0066]
[0067] In a 100mL three-necked round-bottom flask, add 0.90g of the above-mentioned synthesized 9 and 50mL of absolute ethanol, and add 3.50g of SnCl 2 2H 2 O dissolved in concentrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com