Matrix type patch containing bronchodilators
A bronchodilator and matrix technology, which can be used in medical preparations containing active ingredients, medical preparations without active ingredients, drug combinations, etc., and can solve the problems of low skin permeability and low adhesion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
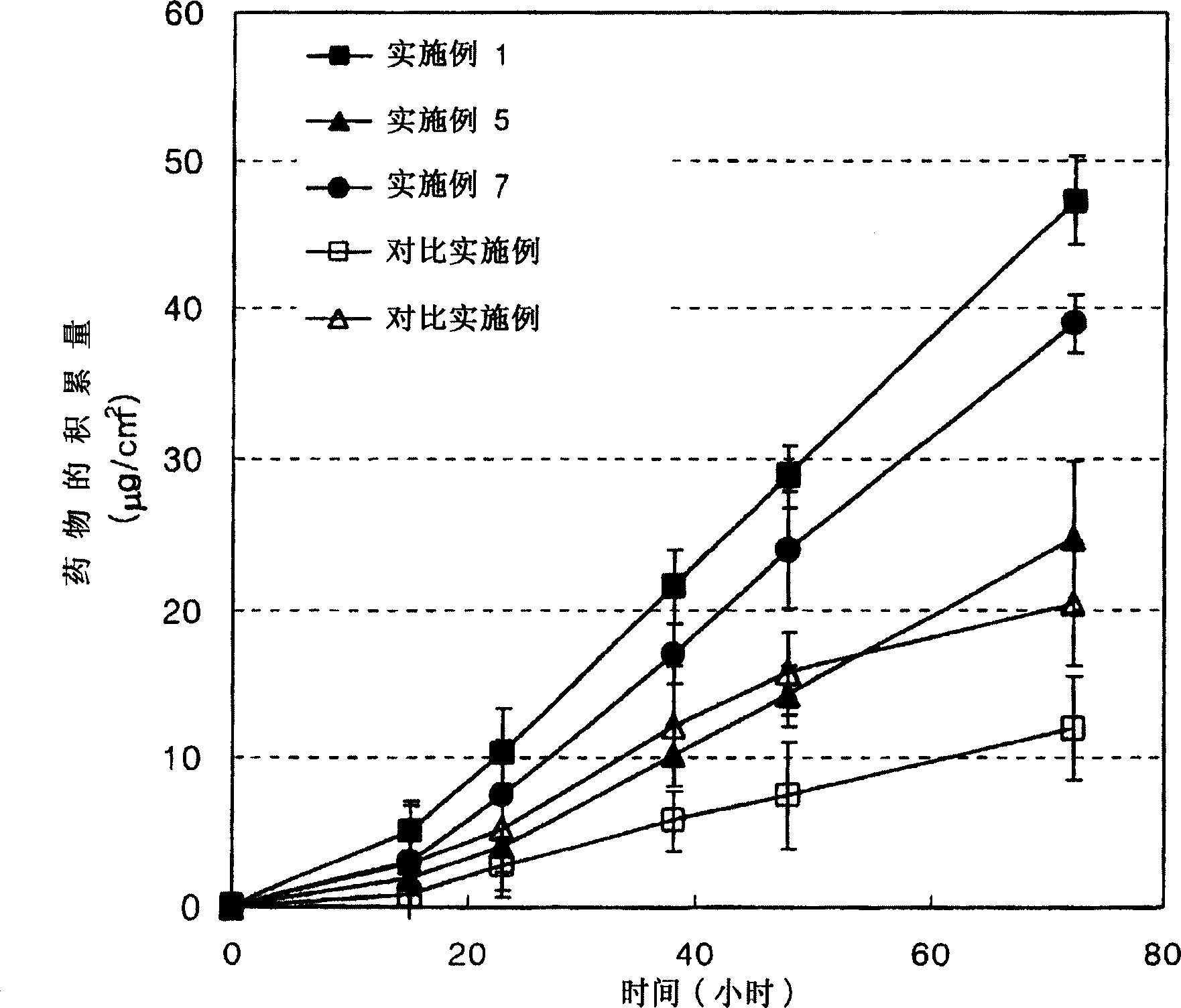
Examples
Embodiment 1
[0040] The matrix-type patch of the present invention is produced as follows.
[0041] 400 mg of formoterol fumarate as a drug was added to 4 ml of methanol and completely dissolved to obtain a homogeneous solution. 1.4 g vinylpyrrolidone-dimethylaminoethyl methacrylate copolymer {poly(vinylpyrrolidone-co-dimethylaminoethyl methacrylate): "Copolymer 958" , manufactured by ISP TECHNOLOGIES, INC., USA} as cationic polymerization absorption accelerator; 2g laurylpyrrolidone and 0.4g triethanolamine as solubilizer; 0.6g vinylpyrrolidone-ethylene vinyl acetate copolymer {Poly(vinylpyrrolidone-co-vinyl vinyl acetate), PVP / VA I-735, I-535, I-335, made by ISPTECHNOLOGIES, INC., U.S.} as a polymer additive to increase viscosity Adhesion and bonding of the mixture layer; and 15.1 g of acrylic adhesive polymer solution "Duro-TAK 87-4098" (manufactured by National Starch Co., U.S.) as a base material with 50 mg of butylated hydroxytoluene (BHT) Together with 50 mg of butylated hydroxyan...
Embodiment 2~9
[0044] The composition (%) of each component used in Examples 2-9 is listed in Table 1, and the procedure is the same as described in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com