Monoclonal antibody of SARS coronavirus N protein and its application
A SARS virus and monoclonal antibody technology, applied in the direction of antiviral immunoglobulin, antibody, antiviral agent, etc., can solve the problems of long time required, high technical difficulty, and high equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1: the expression of SARS N protein
[0077] After inserting the full-length gene of SARS N protein (SEQ ID NO: 1) into the expression vector pETs, transfect Escherichia coli BL21 (DE3). Add IPTG to a final concentration of 0.5mmol / L to induce bacteria to express N protein, then purify by Ni-NTA affinity chromatography according to the operation manual, and then detect by SDS-PAGE.
Embodiment 2
[0078] Embodiment 2: Preparation of polyclonal antibody
[0079] Animal immunization: Rabbits were immunized three times with the purified N protein expressed in Example 1. For the first immunization, use Freund’s complete adjuvant and N protein in equal amounts and then emulsify it, and inject it at multiple points under the skin, 1 mg / cause; for the second and third immunizations, use Freund’s incomplete adjuvant and N protein in equal amounts, and then emulsify , The immunization method and dosage are the same as the first immunization. The interval between each immunization is about one month, and the blood is collected one week after the third immunization.
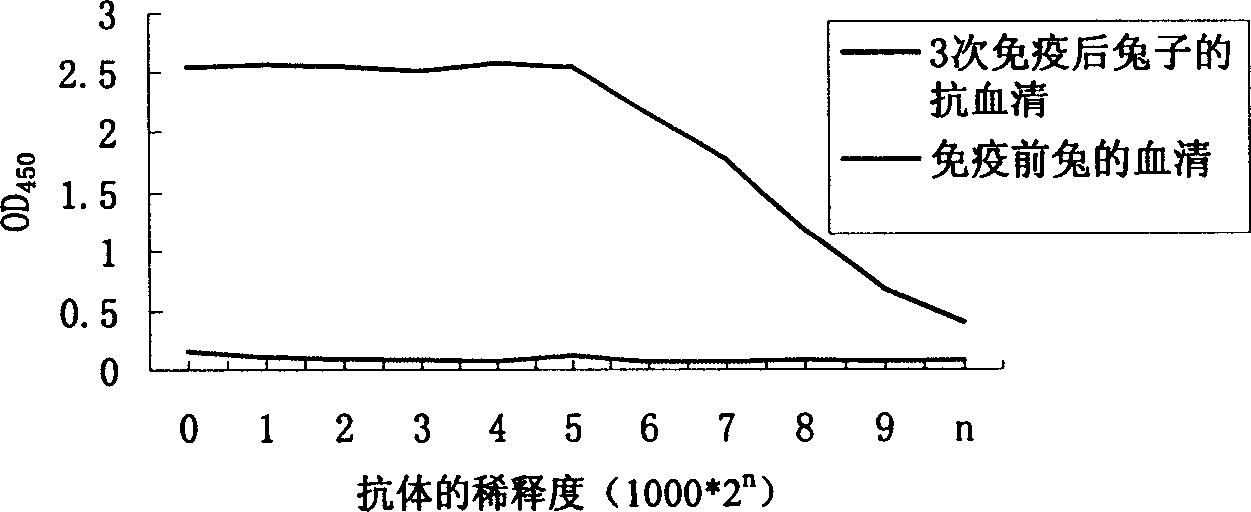
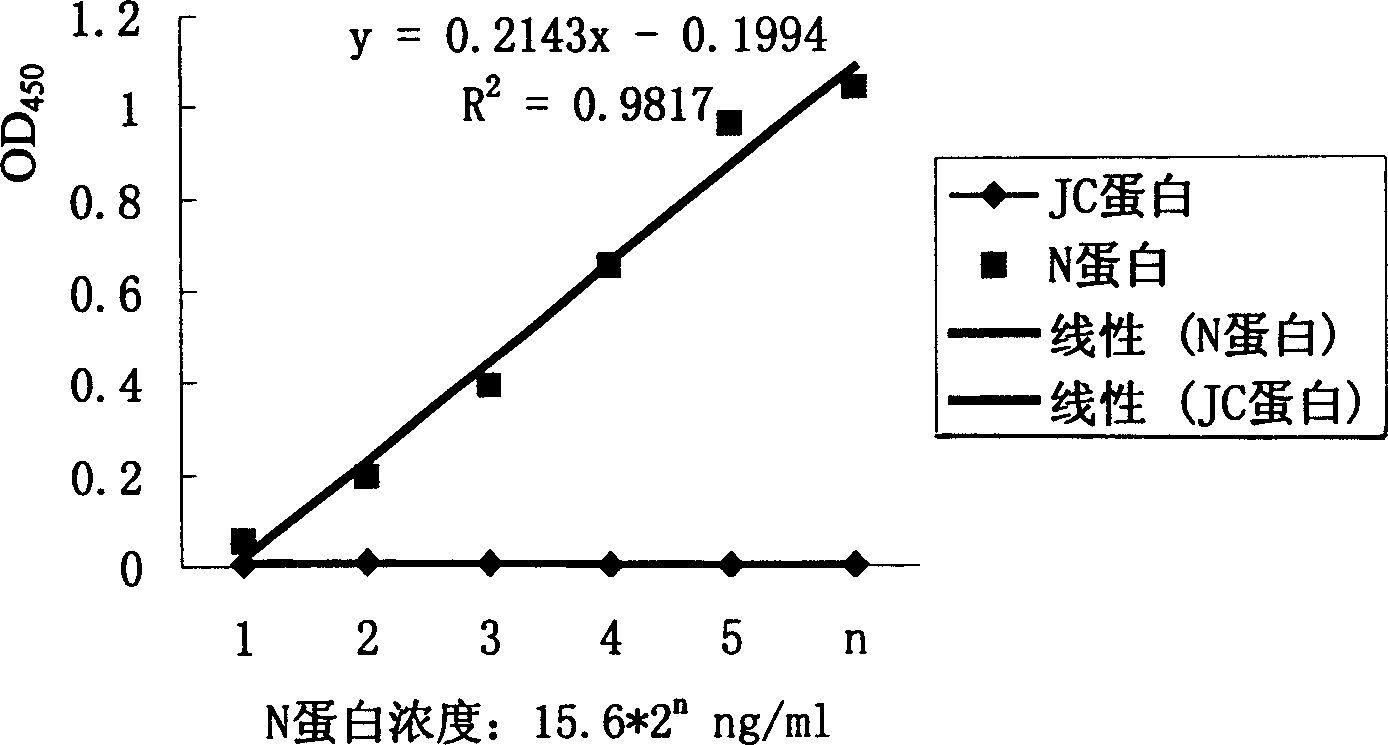
[0080] Indirect enzyme-linked immunosorbent assay (indirect ELISA) to detect the titer of antiserum:
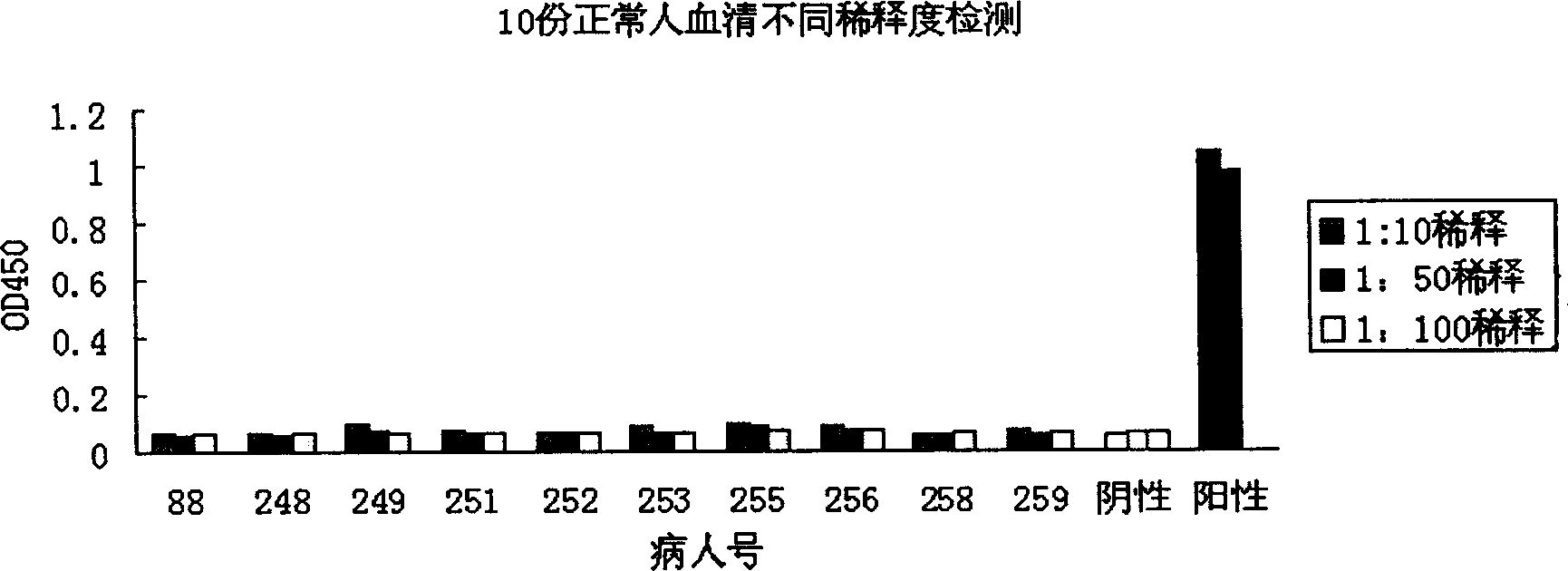
[0081] Coat the ELISA detection plate with N protein, block with PBS blocking solution containing 10% bovine serum and 0.1% Tween, then add different dilutions of antiserum, incubate at 37°C for 2 hours, and set t...
Embodiment 3
[0085] Example 3: Establishment of anti-N protein hybridoma cell lines and preparation of monoclonal antibodies thereof
[0086] 1. Animal immunization: BALB / C mice were immunized with recombinant N protein three times. The immunization method was the same as that of rabbits, and one month after the third immunization, the mice were recalled. The titer of antiserum detected by indirect ELISA was 64000.
[0087] 2. Preparation and screening of hybridomas: Take mouse spleen cells 3 days after memory stimulation and NS-1, SP2 / 0 myeloma cells were fused with 50% PEG according to conventional methods, and after screening and cloning by indirect ELISA method, we obtained Hybridoma cell strains, in which the S-39-2 clone was used in this work (the deposit number is CCTCC NO: -C200407). The antibody subtype secreted by this cell line is IgG1.
[0088] 3. Preparation of ascites and purification of antibodies: each mouse was intraperitoneally injected with 0.5ml pristane, 7-10 days l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com