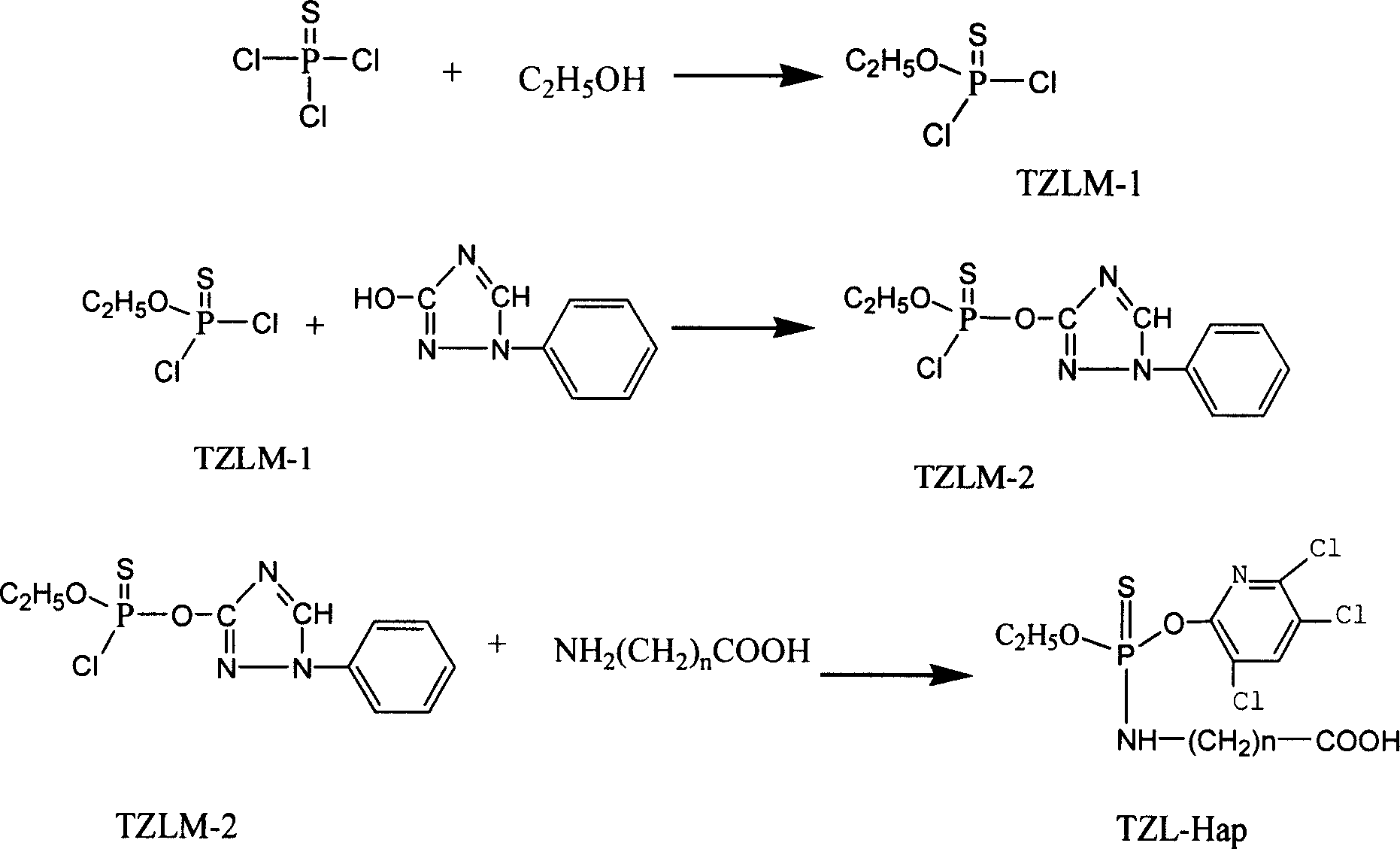
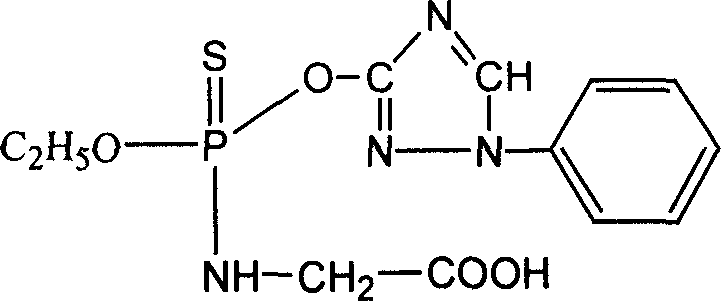
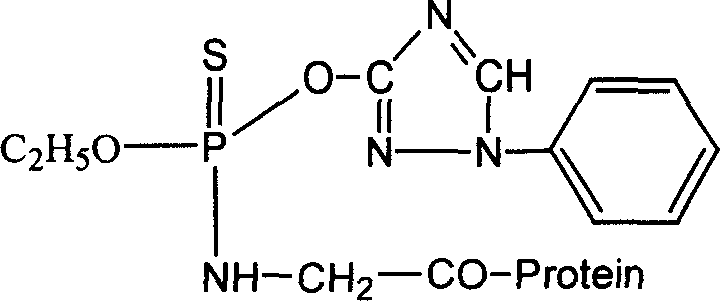
Preparation of triazoline artificial semi-antigen, antigen and antibody
A technology of artificial hapten and triazophos, which is used in the preparation of antibodies and the field of antigens, can solve the problems of cumbersome, complicated and toxic processes, and achieve the effects of high sensitivity, good affinity and low cross-reaction rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Triazophos artificial hapten synthesis method is as follows:
[0038] 1) Synthesis of O-ethylthiophosphoryl dichloride (TZLM-1)
[0039] Weigh the phosphorus trichloride (PSCl 3 ) 68g (about 0.4mol) was placed in a three-necked flask with a low-temperature thermometer, cooled to -10~-5°C with an ice-salt water bath, and 18.4g (about 0.4mol) of absolute ethanol was added dropwise under vigorous stirring, Strictly control the rate of addition so that the temperature of the reaction solution is always at -30 to 0°C. After the dropwise addition was completed, the reaction was continued at -20-10°C for 1 h. After the reaction, the reaction solution (100ml×2) was washed with (0±5)°C distilled water, the oil layer was separated and washed with anhydrous Na 2 SO 4 Dry it, then distill it under reduced pressure with a water pump, and collect fractions at 65-75° C. to obtain 28.5 g of a colorless transparent oily liquid (yield 40%, calculated as phosphorus trichloride).
...
Embodiment 2
[0074] 1. Triazophos artificial hapten synthesis method is as follows:
[0075] 1) Synthesis of O-ethylthiophosphoryl dichloride (TZLM-1)
[0076] Weigh the phosphorus trichloride (PSCl 3 ) 68g (about 0.4mol) was placed in a three-necked flask with a low-temperature thermometer, cooled to -10~-5°C with an ice-salt water bath, and 55g (about 1.2mol) of absolute ethanol was added dropwise under vigorous stirring, strictly Control the rate of addition so that the temperature of the reaction solution is always at -30 to 0°C. After the dropwise addition, the reaction was continued at -20~10°C for 2h. After the reaction is complete, wash the reaction solution (100ml×2) with (0±5)°C distilled water, separate the oil layer and wash it with anhydrous Na 2 SO 4 After drying, it was distilled under reduced pressure by a water pump, and the fraction at 65-75°C was collected to obtain 51.8 g of a colorless transparent oily liquid (yield 72.3%, calculated as phosphorus trichloride).
...
Embodiment 3
[0111] 1. The synthetic method of triazophos artificial hapten is as follows:
[0112] 1) Synthesis of O-ethylthiophosphoryl dichloride (TZLM-1)
[0113] Weigh the phosphorus trichloride (PSCl 3 ) 68g (about 0.4mol) was placed in a three-necked flask with a low-temperature thermometer, cooled to -10~-5°C with an ice-salt water bath, and 92g (about 2.0mol) of absolute ethanol was added dropwise under vigorous stirring, strictly Control the rate of addition so that the temperature of the reaction solution is always at -30 to 0°C. After the dropwise addition, the reaction was continued at -20-10°C for 5h. After the reaction, the reaction solution (100ml×2) was washed with (0±5)°C distilled water, the oil layer was separated and washed with anhydrous Na 2 SO 4 After drying, it was distilled under reduced pressure by a water pump, and the fraction at 65-75°C was collected to obtain 57.0 g of a colorless transparent oily liquid (yield 79.53%, calculated as phosphorus trichloride...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com