Method for manufacturing 2-(hydroxymethyl) cyclopropane carboxylic acid compound
A technology of cyclopropane carboxylic acid and compound, which is applied in the field of preparation of 2-(hydroxymethyl) cyclopropane carboxylic acid compound, and can solve problems such as many steps and complex synthesis of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
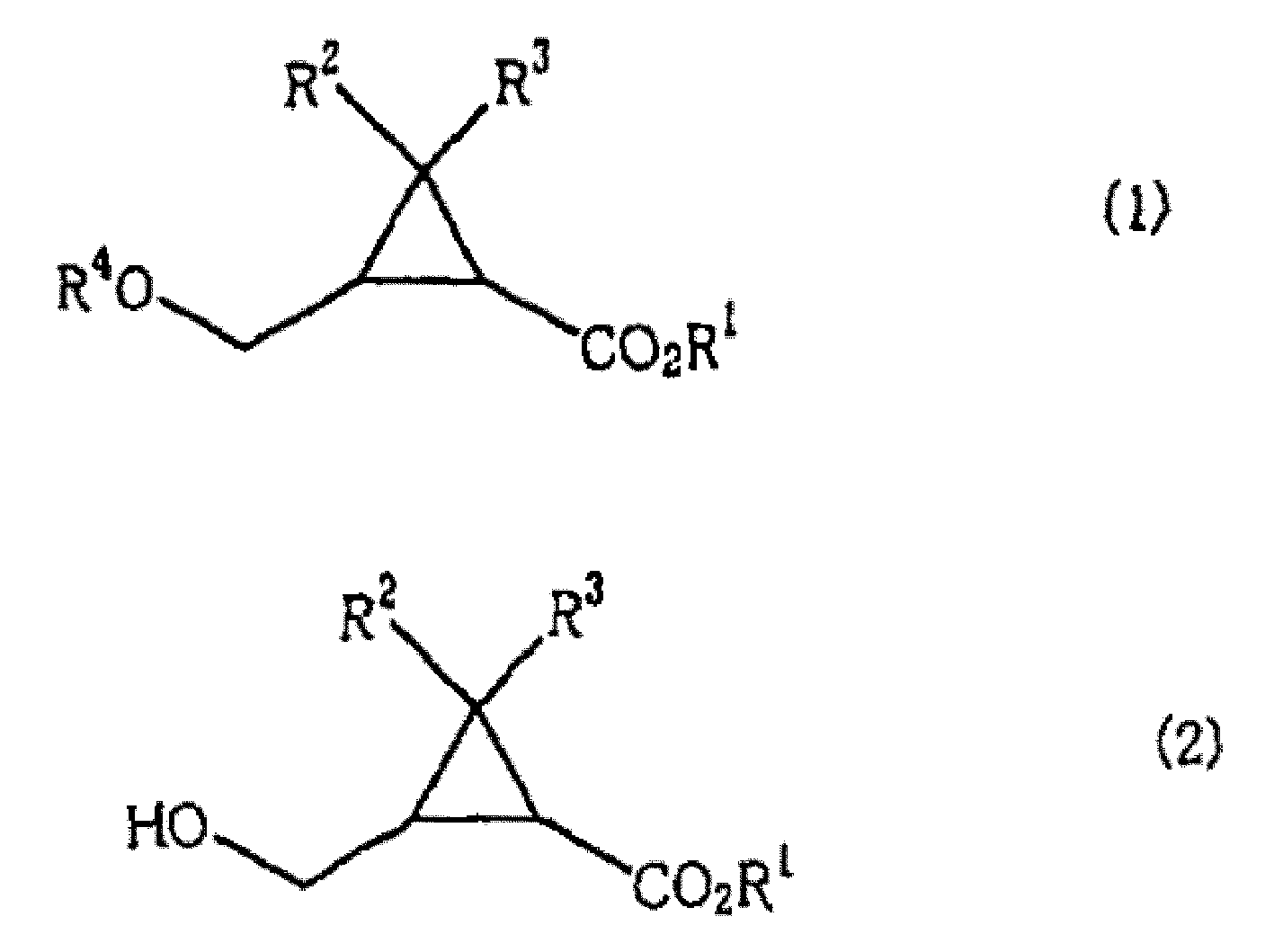
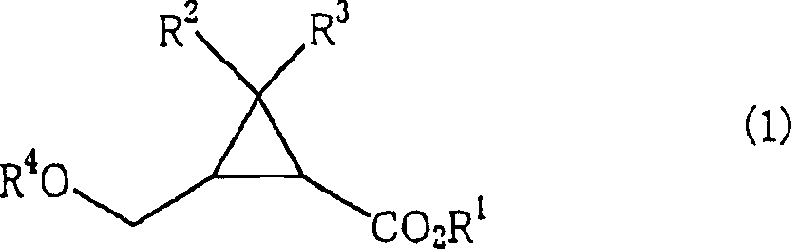
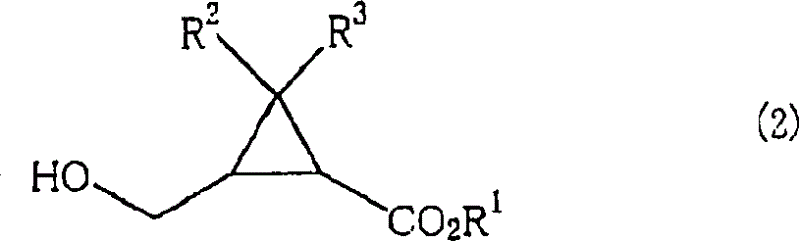
[0067] In the preparation method of the present invention, when using the mixture of the cis-isomer and the trans-isomer as the compound shown in the formula (1), the 2 shown in the formula (2) that keeps the ratio of the cis-isomer / trans-isomer can be obtained. -(hydroxymethyl)cyclopropanecarboxylic acid compound; and when using optically active body as the compound shown in formula (1), can obtain the 2-(hydroxymethyl) shown in the formula (2) that keeps its stereo configuration Optically active form of cyclopropanecarboxylic acid compound.
[0068] Therefore, as the obtained 2-(hydroxymethyl)cyclopropanecarboxylic acid compound represented by the formula (2), for example, 2-(hydroxymethyl)cyclopropanecarboxylic acid, 2-(hydroxymethyl)cyclopropanecarboxylic acid methyl Ester, ethyl 2-(hydroxymethyl)cyclopropanecarboxylate, tert-butyl 2-(hydroxymethyl)cyclopropanecarboxylate, cyclohexyl 2-(hydroxymethyl)cyclopropanecarboxylate, 2-(hydroxymethyl)cyclopropanecarboxylate Methyl...
Embodiment 1
[0075] Into a 200 mL Schlenk tube were added 2.6 g of ethyl 2-(benzyloxymethyl)-3,3-dimethylcyclopropanecarboxylate, 100 mL of 4.4% by weight formic acid / methanol solution, and 10% by weight of palladium / carbon 2.6 g, stirred at room temperature for 7 hours to react. Then, the reaction solution was filtered through diatomaceous earth to remove the catalyst, and then the obtained filtrate was concentrated to obtain ethyl 2-(hydroxymethyl)-3,3-dimethylcyclopropanecarboxylate in the form of a colorless transparent oil 1.7 g, yield 98%.
Embodiment 2
[0077] Under a nitrogen atmosphere, 193 g of a 23% aqueous sodium hydroxide solution was added to a 1 L four-necked flask, and heated to 110°C. To this aqueous solution, 222 g of a solution containing 48.9% of ethyl 2-(benzyloxymethyl)-3,3-dimethylcyclopropanecarboxylate was added dropwise over 3.5 hours. Hour. After cooling to room temperature, 333 ml of water and 111 ml of toluene were added for liquid separation to obtain an aqueous solution containing crude 2-(benzyloxymethyl)-3,3-dimethylcyclopropanecarboxylic acid sodium salt. Repeat the above liquid separation operation three times. 127 g of 35% hydrochloric acid and 111 ml of toluene were added to the obtained aqueous solution for liquid separation to obtain a toluene solution containing crude 2-(benzyloxymethyl)-3,3-dimethylcyclopropanecarboxylic acid. Repeat the above liquid separation operation three times. The obtained toluene solution was washed three times with 111 ml of water, and then concentrated to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com