Nano-magnetic medicinal microglobule, its preparation method and application
A nano-magnetic and nano-magnetic material technology, applied in the application field of medicine, can solve problems such as hindering application and low drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
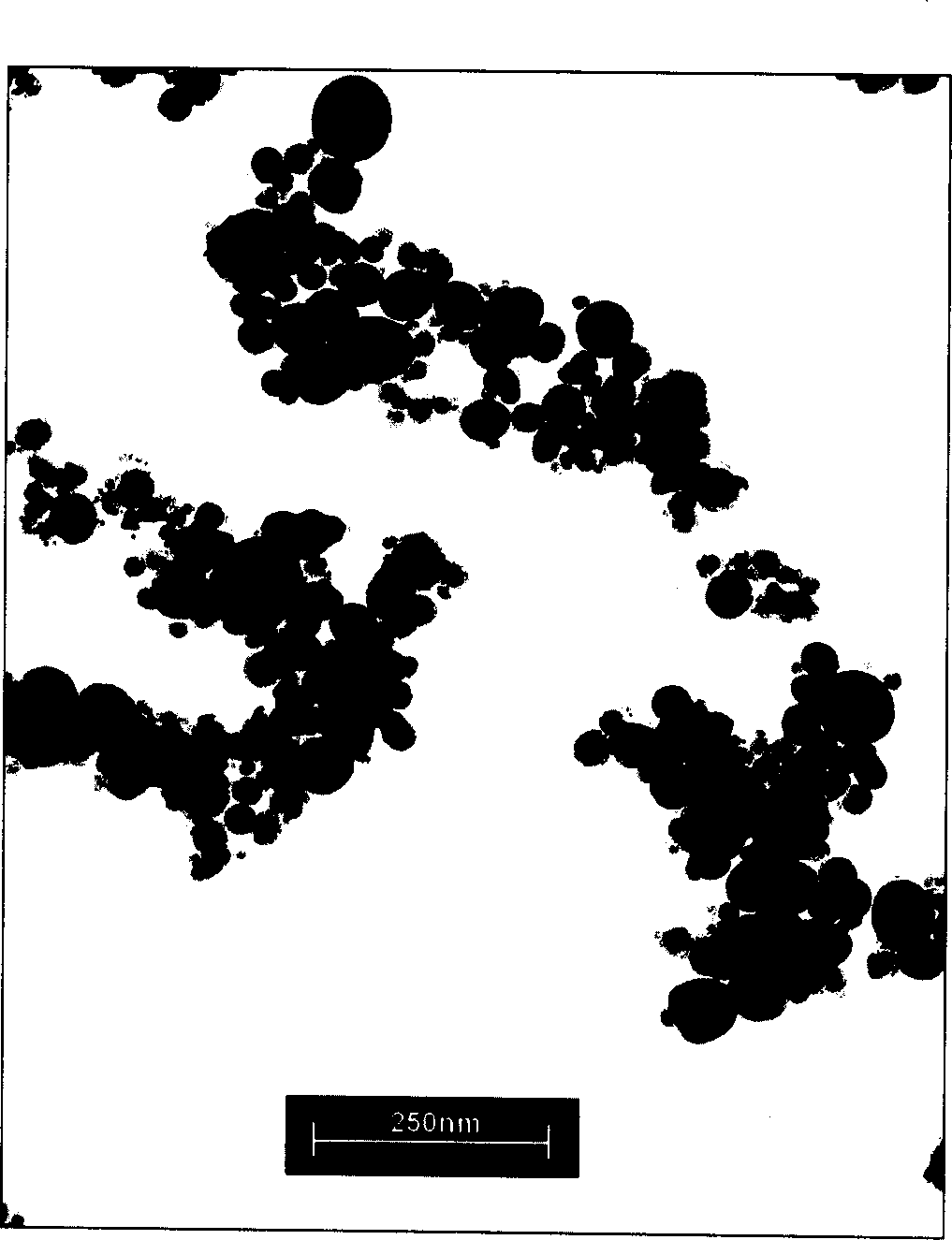
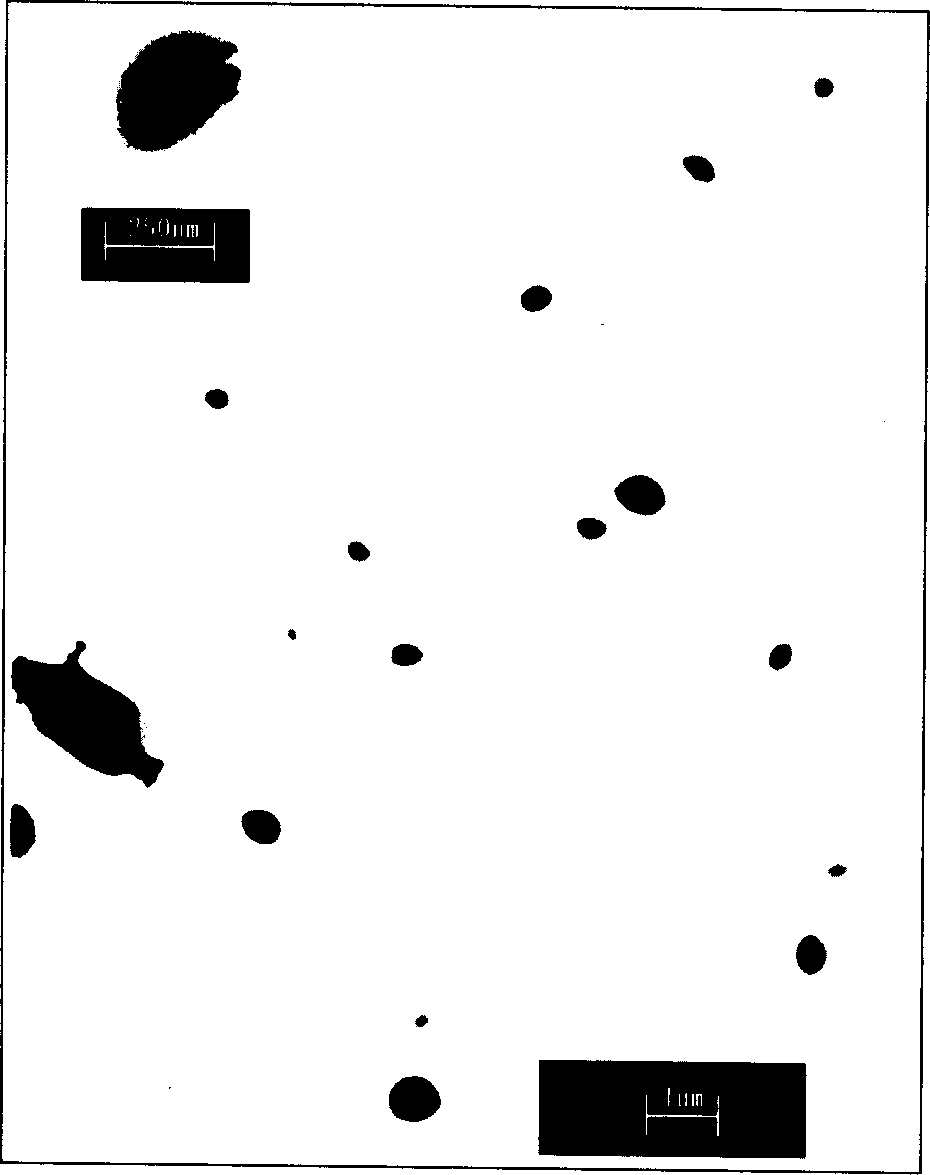
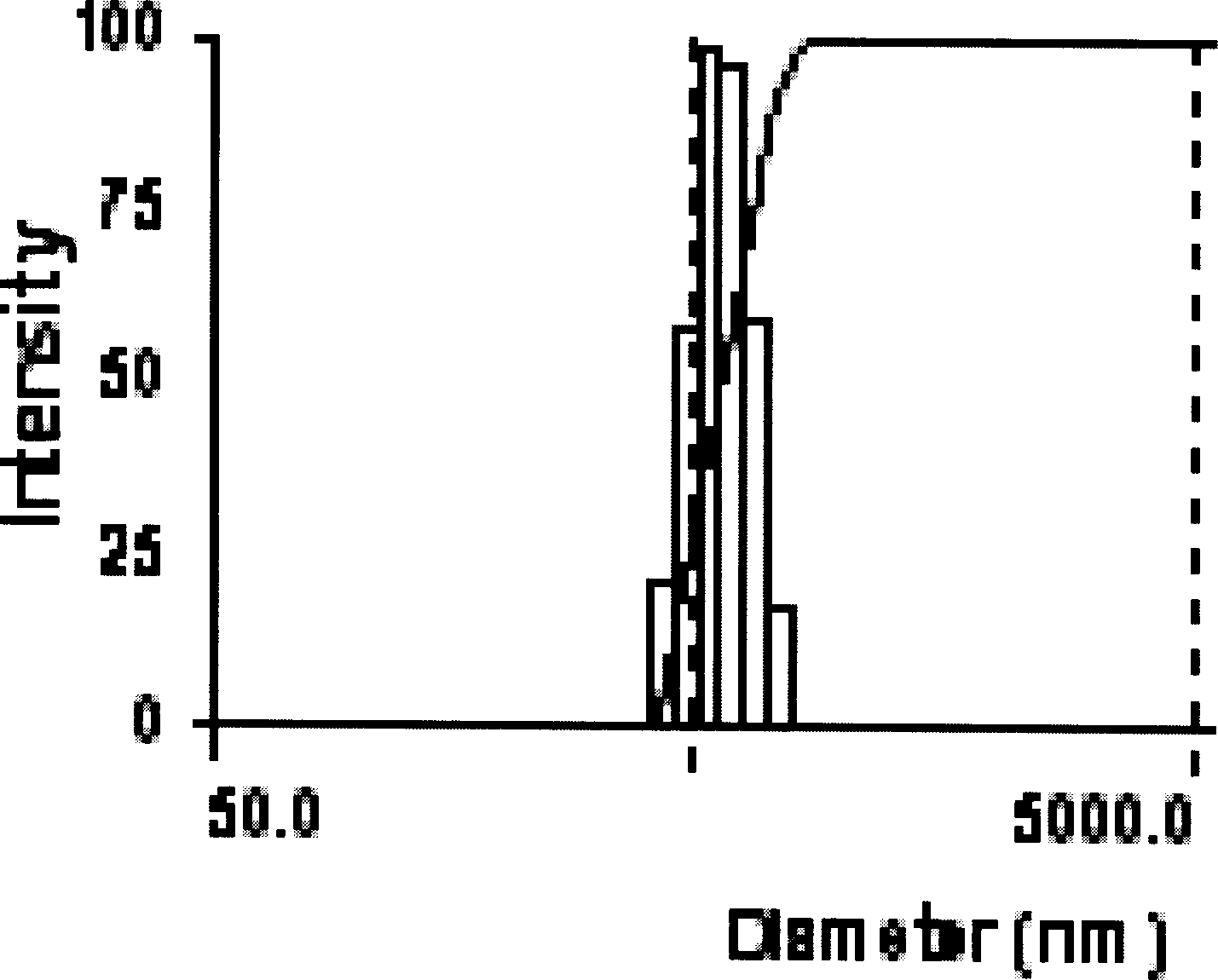
[0071] Implementation Proceeding 1: Take 34.8mg carbon-coated iron-ferromagnetic powder (produced by Shenzhen Zunye Nano Materials Co., Ltd., model is JY-FeC, average particle size is 25nm, maximum particle size is 60nm), magnetic carbon-coated iron powder transmission electron microscope See figure 1 . 32.4mg of doxorubicin (produced in Japan, provided by Shenzhen Wanle Pharmaceutical Co., Ltd., content 98.4%), 31.0mg chitosan (provided by Jinan Haidebei Marine Bioengineering Company, deacetylation degree > 95.7%) was dissolved in 3mlHAC ( 5%), ultrasonically mixed, placed to remove air bubbles, and then 0.6ml of 5% glutaraldehyde aqueous solution was added, ultrasonically mixed to form an aqueous suspension. Above-mentioned suspension is dripped in 30ml toluene, wherein comprises 12gAOT (the AOT of described, is aerosol OT, and molecular formula is C 20 h 37 NaO 7 S, the molecular weight is 444.56, purchased by Shanghai Jufeng Chemical Technology Co., Ltd., produced by J...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com