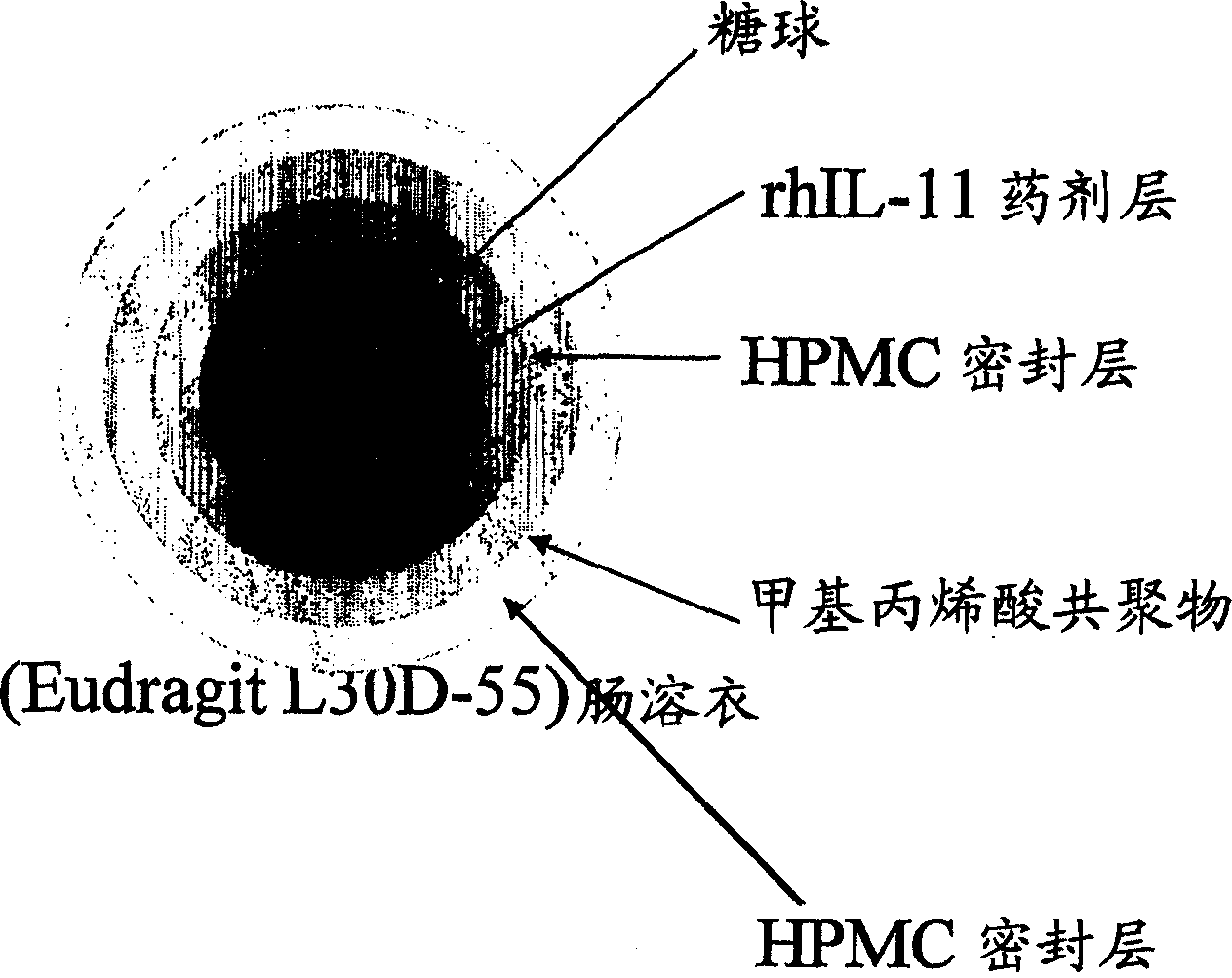
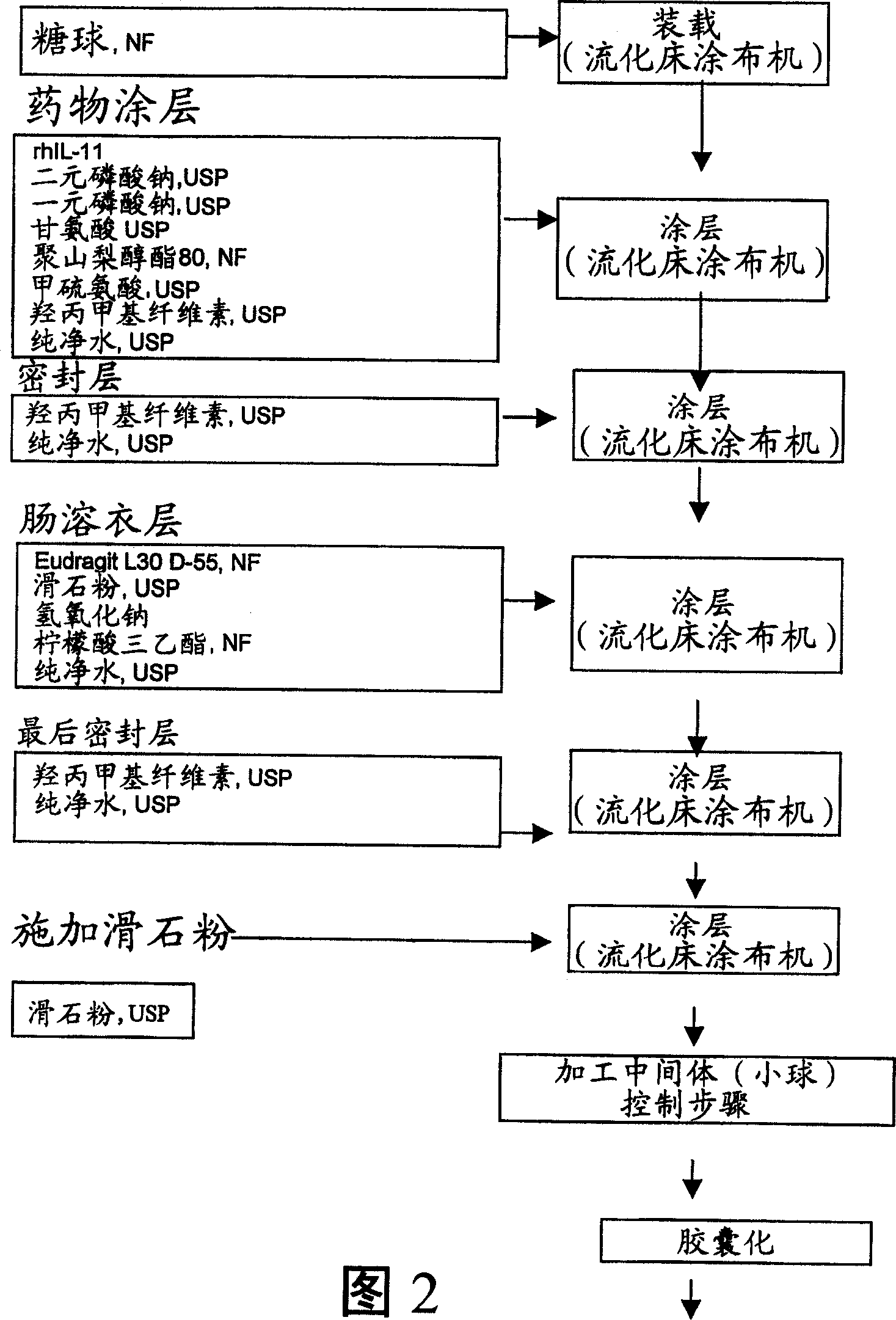
Delayed release formulations for oral administration of a polypeptide therapeutic agent and methods of using same
A slow-release, therapeutic effect technology, applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, antipyretics, etc., can solve problems such as inconvenient administration, high price, and discomfort of the subjects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1.rhIL for compatibility of various formulation excipients and antioxidants
[0051] Compatibility studies were performed on rhIL-11 tablets containing formulation excipients and antioxidants listed in Table 1. Excipients studied included fillers, disintegrants, buffers, glidants, and lubricants. A rhIL-11 tablet containing the above-mentioned excipients was prepared by direct compression. The lyophilized rhIL-11 was collected, filtered through a #30 mesh sieve, and transferred to a suitable sized vial containing all other excipients. Mix the material by swirling the vial for 2-3 minutes. For formulations including magnesium stearate (F1, F2, F4-F8), the magnesium stearate was added at this point and mixing continued for an additional 0.5-1 minute.
[0052] Each tablet weighs 150 mg and contains 2.5 mg of rhIL-11 (addition of lyophilized powder prepared from a freeze-dried vial freeze-dried concentrate containing rhIL-11 in quantities equivalent to 5 mg alon...
Embodiment 2
[0055] Example 2: Integrity of rhIL-11 capsules during tablet preparation
[0056] Integrity of rhIL-11 under applied pressure was checked during tablet processing. Different pressures were used to evaluate the effect of tablet manufacturing pressure on rhIL-11 integrity. These tablets weigh 150 mg and contain 2.5 mg of rhIL-11 (lyophilized powder), EXPLOTAB(R), microcrystalline cellulose, NU-TAB(R), silicates and magnesium stearate. Tablets are directly compressed to a hardness of 2.4, 4.0, 7.5 or 12.5 KP. By determining the recovery %, multibody %, Met 58 Protein integrity can be determined by % oxidation, % correlation, and specific activity of rhIL-11 by T-10 bioassay. The test results in Table 4 show that for rhIL-11 tablets compressed into different hardness levels, recovery %, multibody %, Met 58 Oxidation %, correlation % did not change. Similarly, the specific activities of the different formulation mixtures and tablets were within the range stated in the label...
Embodiment 3
[0057] Example 3: Stability of enteric-coated rhIL-11 tablets
[0058] The stability of enteric-coated tablets prepared by the fluidized bed granulation method was determined using HDPE bottles at 40°C / 75%RH and room temperature. Stability experiments include Recovery %, Met 58 Determination of % Oxidation, % Correlation. The measurement results are summarized in Table 6. When stored at room temperature and 40°C / 75%RH, the strength of rhIL-11, Met 58 Oxidation %, relative % did not change.
[0059] In the micro-dissolution device, the dissolution experiment was carried out using 50 ml of glycine / phosphate dissolution medium with stirring speed at 50 or 100 rpm. The coated tablets were first placed in 0.1N HCl for 2 hours, and then placed in glycine / phosphate dissolution medium for 1 hour, and then the release of rhIL-11 from the coated tablets was detected. The dissolution results showed that less than 1% of rhIL-11 was released within two hours in 0.1 N HCl. This show...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com