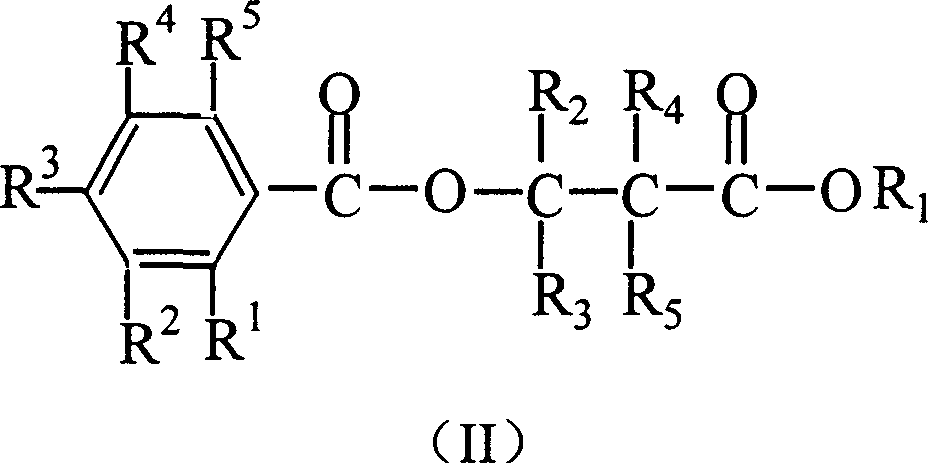
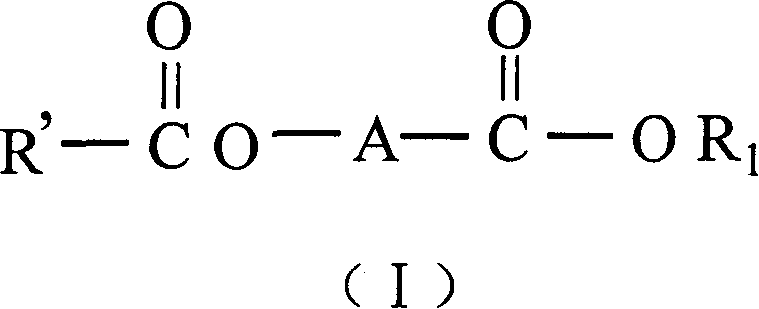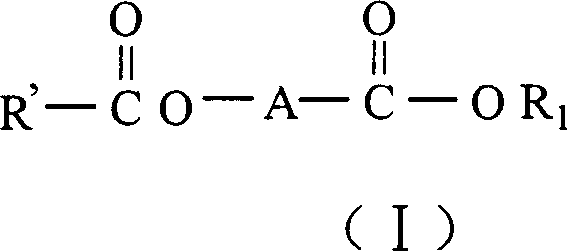Catalyst components for olefinic polyreaction and catalyst thereof
A technology for olefin polymerization and catalyst, applied in the field of catalyst components and catalysts for olefin polymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: Preparation of ethyl 2-benzyl-3-benzoyloxybutyrate
[0064] (1) Preparation of ethyl 2-benzyl acetoacetate
[0065] 0.1mol ethyl acetoacetate, 0.1mol K 2 CO 3 ., 0.1 mol benzyl bromide, 0.01 mmol PEG-400 (polyethylene glycol 400) and 100 ml benzene were stirred at 75° C. for 7 hours. .Add 20ml NH after cooling 4 Dissolve in a saturated Cl solution and extract with ethyl acetate. Remove the solvent, distill under reduced pressure, and collect the fraction 116-118°C / 20Pa. The yield was 74%.
[0066] (2) Preparation of ethyl 2-benzyl-3-hydroxybutyrate
[0067] 0.05mol NaBH 4 25ml of water was added to 0.4g of NaOH, cooled in an ice bath, a mixture of 0.07mol ethyl 2-benzyl acetoacetate and 30ml of methanol was added dropwise with stirring, and stirred at room temperature for 5 hours. Remove solvent, extract with ethyl acetate, anhydrous Na 2 SO 4 After drying, the solvent was removed to obtain 0.06mol of a colorless liquid. Yield 85%.
[0068] (3)...
Embodiment 2
[0078] Embodiment 2: the preparation of ethyl 3-benzoyloxybutyrate
[0079] 1) Preparation of ethyl 3-hydroxybutyrate
[0080]In a three-neck flask equipped with a titration device, add 1.5g of sodium borohydride, 0.02g of sodium hydroxide, and 13ml of water in sequence, and stir to mix evenly. In an ice-water bath, slowly add a mixture of 0.1 mol of ethyl acetate and 15 ml of anhydrous methanol dropwise into the reaction flask under stirring conditions. Continue to react for 2 hours after dropping. The solvent methanol and most of the water were evaporated to dryness using a rotary evaporator until the residue was a solid phase. Extract with anhydrous diethyl ether for 24hr under stirring condition. The extract was filtered and dried over anhydrous sodium sulfate. The solvent was evaporated to dryness to obtain 0.052 mol of the product ethyl 3-hydroxybutyrate. Yield 52%.
[0081] 2) Preparation of ethyl 3-benzoyloxybutyrate
[0082] Under anhydrous and oxygen-free nitr...
Embodiment 3
[0090] Embodiment 3: Preparation of ethyl 2-methyl-3-benzoyloxybutyrate
[0091] 1) Preparation of ethyl α-methyl acetoacetate
[0092] Under anhydrous and oxygen-free nitrogen protection conditions, add 0.15mol potassium tert-butoxide and 150ml tetrahydrofuran in sequence to a three-necked flask equipped with a titration device, and start stirring. Under the condition of ice-water bath, 0.12mol ethyl acetoacetate was slowly added dropwise. After the dropwise addition, the reaction was continued for 1 hr at room temperature. Slowly add 0.18mol iodomethane dropwise at room temperature, after the dropwise addition, continue to react at room temperature for 24 hours. After the reaction was completed, evaporate the solvent to dryness with a rotary evaporator, add saturated saline until the solid mixture was just completely dissolved, separate the organic phase, extract the aqueous phase with an appropriate amount of anhydrous ether three times, combine the organic phases, wash f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melt flow index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com