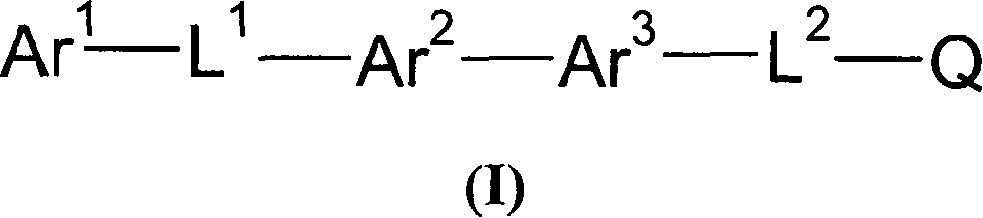Novel MCH receptor antagonists
A CH2, enantiomer technology, used in the medical field, can solve problems such as unproven patient efficacy and long-term efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment
[0395] Preparation 1
[0396] tablet
[0397] Ingredient Quantity (mg / tablet)
[0398] Active ingredient 5-500
[0399] Microcrystalline cellulose 200-650
[0400] Fumed silica 10-650
[0401] Stearic acid 5-15
[0402] The ingredients are blended and compressed into tablets.
[0403] Preparation 2
[0404] Suspension
[0405] Component Quantity (mg / 5ml)
[0406] Active ingredient 5-500mg
[0407] Sodium Carboxymethyl Cellulose 50mg
[0408] Syrup 1.25mg
[0409] Benzoic acid solution 0.10mL
[0410] Flavoring q.v.
[0411] pigment q.v.
[0412] Purified water, to 5mL
[0413] The active ingredient is passed through a No. 45 mesh U.S. sieve (approximately 355 micron openings) and mixed with the sodium carboxymethylcellulose and syrup to form a homogeneous paste. The benzoic acid solution, flavor and color are diluted with some of the water and added with stirring. Sufficient water is then added to achieve the desired volume.
[0414] Preparation 3
[04...
Embodiment 1
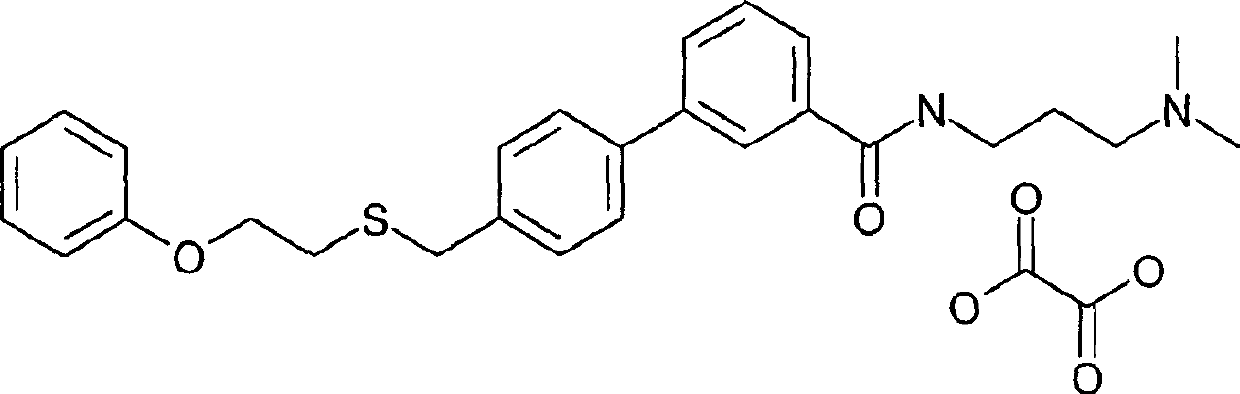
[0473] Preparation of 4'(2-phenoxy-ethylthiomethyl)-biphenyl-3-carboxylic acid (3-dimethylamino-propyl)-amide oxalate
[0474]
[0475] A solution of 4'-(2-phenoxy-ethylthiomethyl)-biphenyl-3-carboxylic acid (0.91 g, 2.5 mmol, 1 eq.) in anhydrous THF (10 mL) was treated with 1,1'-carbonyl di Imidazole (0.41 g, 2.55 mmol, 1.02 eq.) was treated and the resulting solution was heated to 60° C. for 25 minutes. The solution was then allowed to cool and 3-(dimethylamino)propylamine (0.31 g, 0.38 mL, 3 mmol, 1.2 eq.) was added via syringe. The reaction was stirred at room temperature. After 2 hours, the reaction was diluted with water and extracted 2 x 150 mL with EtOAc. The combined organic layers were washed with MgSO 4 Dry, filter, and remove the solvent in vacuo to leave 4'-(2-phenoxy-ethylthiomethyl)-biphenyl-3-carboxylic acid (3-dimethylamino-propyl)-amide (0.97 g , 87% yield), as a yellow oil. 4'-(2-Phenoxy-ethylthiomethyl)-biphenyl-3-carboxylic acid (3-dimethylamino-pr...
Embodiment 2
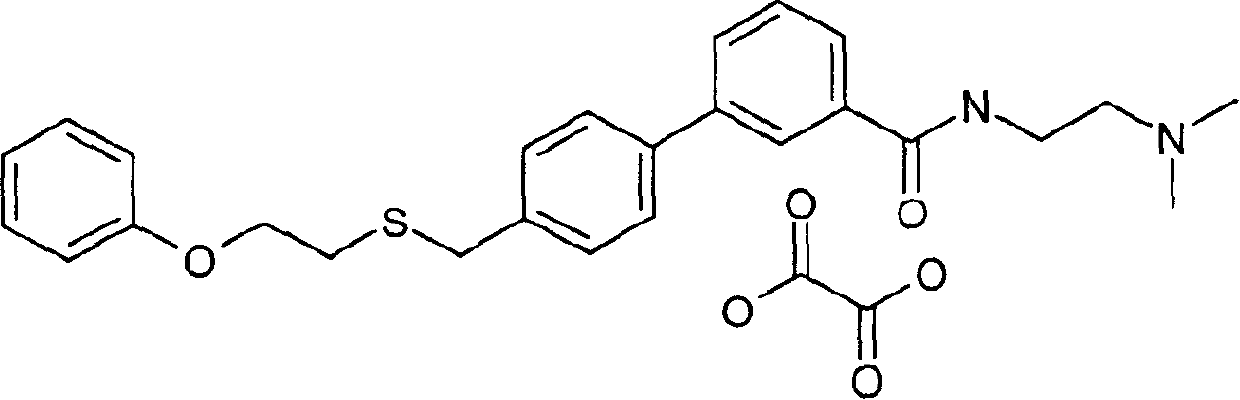
[0478] Preparation of 4'-(2-phenoxy-ethylthiomethyl)-biphenyl-3-carboxylic acid (2-dimethylamino-ethyl)-amide oxalate
[0479]
[0480] Prepared in the same manner as described in Example 1. A solution of 4'-(2-phenoxy-ethylthiomethyl)-biphenyl-3-carboxylic acid (0.68 g, 1.87 mmol, 1 eq.) was treated with 1,1'-carbonyldiimidazole (0.31 g , 1.91 mmol, 1.02 eq.) was treated and heated. The reaction was allowed to cool then treated with N',N-dimethylethylenediamine (0.20 g, 2.24 mmol, 1.2 eq.). The reaction was worked up as described in Example 1 to afford 4'-(2-phenoxy-ethylthiomethyl)-biphenyl-3-carboxylic acid (2-dimethylamino-ethyl)-amide (0.76 g, 94% yield) as a yellowish oil. Conversion of the free base to the oxalate salt as described in Example 1 using 0.20 g of oxalic acid afforded 4'-(2-phenoxy-ethylthiomethyl)-biphenyl-3-carboxylic acid (2-dimethylamino- Ethyl)-amide oxalate (0.5584 g) as a white solid.
[0481] 1 H NMR (DMSO-d6) δ8.89 (br, 1H), 8.14 (s, 1H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com