Bis (3-alkoxyalkan-2-ol) sulfides, sulfones, and sulfoxides: new surface active agents
一种表面活性剂、化合物的技术,应用在表面活性剂组合物领域,能够解决应用复杂等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
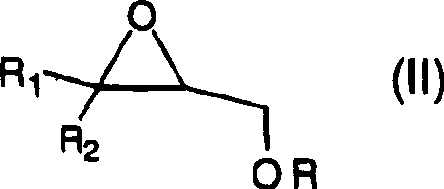
[0018] The preparation of formula (I) compound
[0019] Compounds of formula (I) may be prepared by any method known in the art of synthetic organic chemistry. In an exemplary embodiment of the present invention, it can be prepared by reacting a sulfide source with a glycidyl ether of formula (II), wherein R, R 1 and R 2 As defined above, and where Z=S. Z is SO or SO 2 The compounds of can be prepared by oxidation of the corresponding Z is S compound using oxidation techniques known in the art. In an exemplary embodiment of the present invention, hydrogen peroxide is used for the oxidation reaction, although other methods can also be used.
[0020]
[0021] As used herein, the term "sulfide source" means a composition containing or otherwise providing hydrogen sulfide, a hydrosulfide anion or a sulfide anion. Examples of suitable non-limiting sources of sulfide include compounds M 2 S, wherein M is independently selected from H, NH 4 , alkali metals and alkaline eart...
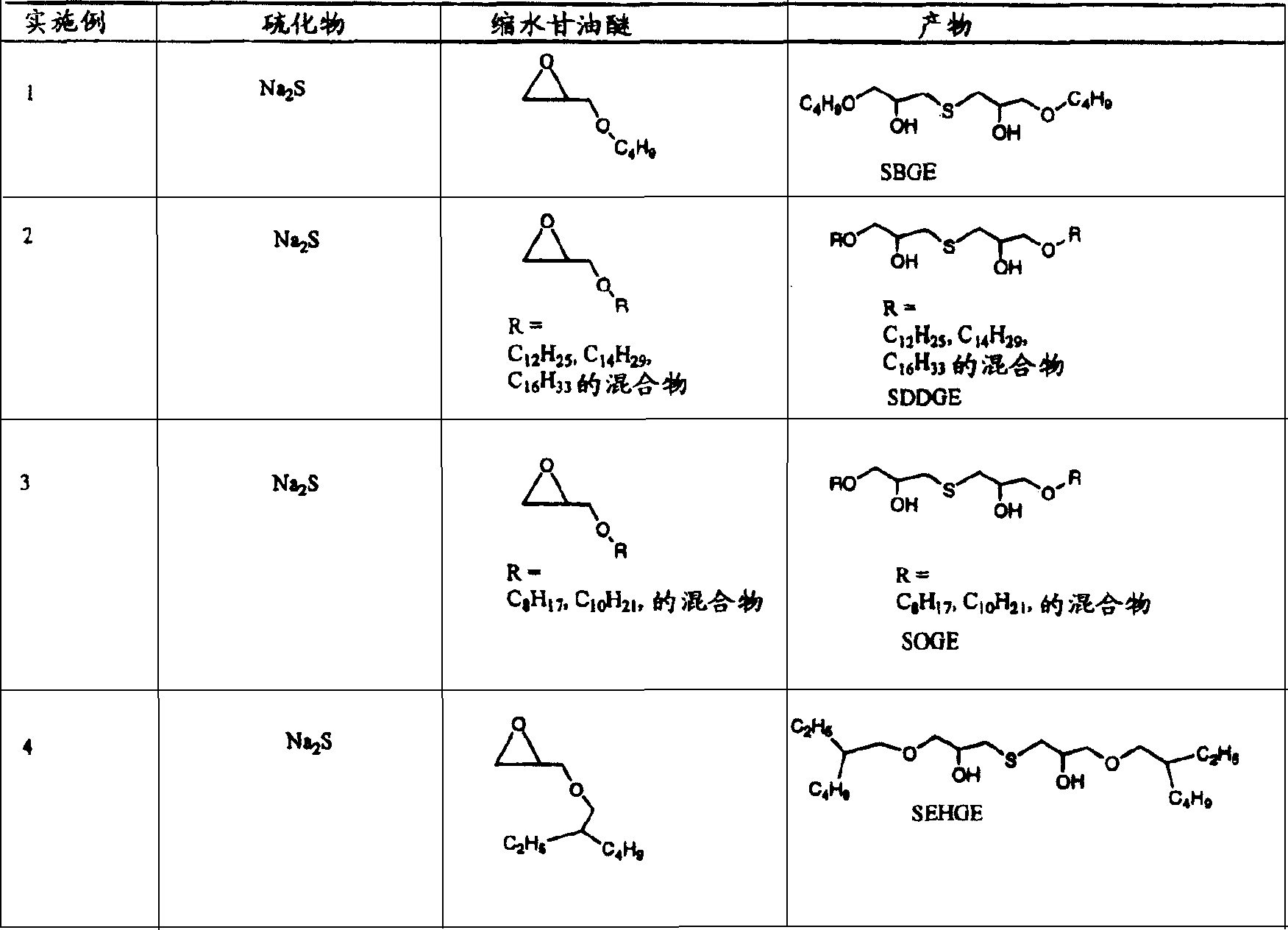
Embodiment 1
[0087] The reaction of embodiment 1-sodium hydrosulfide with butyl glycidyl ether
[0088] Under the condition of nitrogen filling, it is filled with N 2 Butyl glycidyl ether (2.91 g, 22.39 mmol) in isopropanol (5 mL) and H 2 To a solution of O (1 mL) was added sodium hydrosulfide (0.628 g, 11.20 mmol). The mixture was heated at 90°C and completion was monitored by gas chromatography / mass spectrometry for disappearance of reactants and formation of products. After 3 hours, the reaction was deemed complete. The mixture was cooled to ambient temperature and washed with saturated NH 4 Worked up with Cl (5.0 mL) and extracted into ethyl acetate (50 mL). The solvent was dried (MgSO 4 ), filtration and vacuum evaporation yielded the product, 1,1'-thiobis(3-butoxypropan-2-ol), which was compared with 1 H and 13 Confirmed by C NMR.
[0089] The reaction of embodiment 1a-sodium sulfide with butyl glycidyl ether
[0090] Starting from butyl glycidyl ether (2.91g, 22.39mmol) and...
Embodiment 2
[0091] The reaction of embodiment 2-sodium sulfide with C12, C14 and C16 glycidyl ether mixture
[0092] Starting from sodium sulfide (22.48 g, 288 mmol) and a mixture of C12, C14 and C16 glycidyl ethers (139.62 g, ~576 mmol) in 100 mL of isopropanol and 47 mL of water, a procedure similar to that described in Example 1 was followed Carry out the reaction, and the obtained product is 1,1'-thiobis(3-dodecyloxypropan-2-alcohol), 1,1'-thiobis(3-tetradecyl) as determined by the method in Example 1 alkoxypropan-2-ol), 1,1'-thiobis(3-hexadecyloxypropan-2-ol) and have a dodecyloxy group and a tetradecyloxy group, a A mixture of three similar species of hexadecyloxy and one tetradecyloxy, and one hexadecyloxy and one dodecyloxy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com