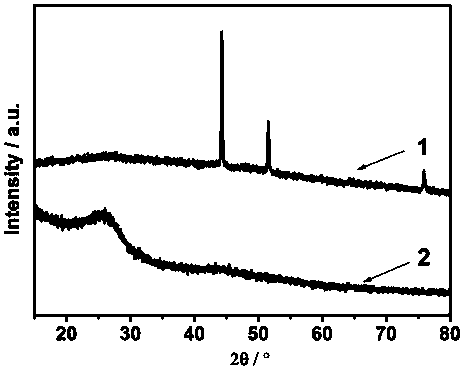
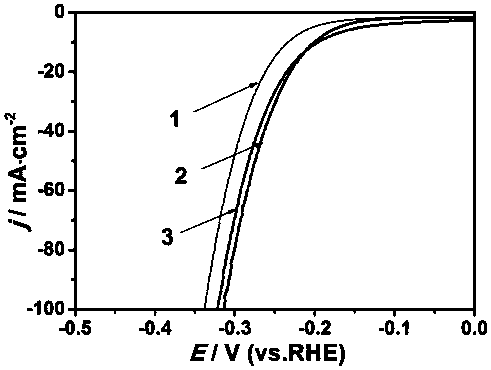
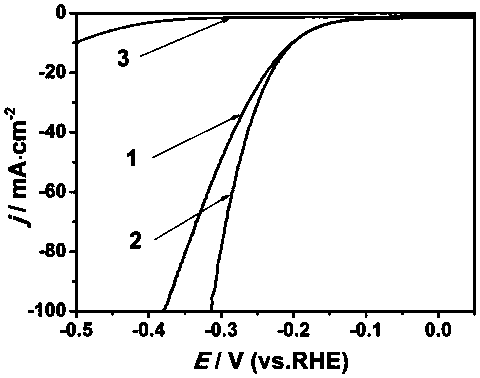
Preparation method of mono-atomic cobalt based nitrogen-sulfur dually-doped carbon material catalyst
A catalyst, double-doping technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve problems such as expensive precursors, achieve simple and easy methods, significant cost advantages, and improve dispersion. Effects of Sexual and Intrinsic Activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Preparation of precursor
[0030] According to the mass ratio of urea: glucose 60: 1, the total mass of urea glucose: the mass ratio of cobalt chloride hexahydrate is 30: 1, the molar ratio of cobalt chloride hexahydrate: sodium thiocyanide is 1: 2, and the urea is weighed , glucose, cobalt chloride hexahydrate, sodium thiocyanate; the above mixture was dissolved in 30-50mL of absolute ethanol, ultrasonically stirred for 5-10 minutes, placed in a blast drying oven at 40°C-60°C, and dried for 4 ~6h, until the ethanol is completely volatilized, and the mixture is recrystallized in a beaker. Then the recrystallized solid was taken out and placed in a ball mill with a rotation speed of 600rpm-800rpm and a number of turns of 2 to obtain a uniform catalyst precursor of urea, glucose, cobalt chloride hexahydrate and sodium thiocyanate.
[0031] (2) Preparation of single-atom cobalt-based nitrogen-sulfur double-doped carbon catalyst (CoNSG1)
[0032] First, place the abov...
Embodiment 2
[0037] (1) Preparation of precursor
[0038] According to the mass ratio of urea:glucose 60:1, the mass ratio of urea glucose:cobalt chloride hexahydrate is 240:1, the molar ratio of cobalt chloride hexahydrate:sodium thiocyanate is 1:2, weigh urea, glucose , cobalt chloride hexahydrate, sodium thiocyanate; dissolve the above mixture in 30-50mL of absolute ethanol, stir ultrasonically for 5-10 minutes, place in a blast drying oven at 40°C-60°C, and dry for 4-6h , until the ethanol was completely evaporated, and the mixture was recrystallized in a beaker. Then the recrystallized solid was taken out and placed in a ball mill with a rotation speed of 600rpm-800rpm and a number of turns of 2 to obtain a uniform catalyst precursor of urea, glucose, cobalt chloride hexahydrate and sodium thiocyanate.
[0039] (2) Preparation of single-atom cobalt-based nitrogen-sulfur double-doped carbon catalyst (CoNSG2)
[0040] First, place the above-prepared precursor in a tube furnace, raise ...
Embodiment 3
[0053] (1) Preparation of precursor
[0054] According to the mass ratio of urea:glucose 60:1, the mass ratio of nitrogen-carbon precursor:cobalt chloride hexahydrate is 360:1, the molar ratio of cobalt chloride hexahydrate:sodium thiocyanate is 1:2, and the nitrogen Carbon precursor, cobalt chloride hexahydrate, sodium thiocyanate; dissolve the above mixture in 30-50mL of absolute ethanol, stir ultrasonically for 5-10 minutes, place in a blast drying oven at 40°C-60°C, and dry 4 ~ 6h, until the ethanol is completely volatilized, and the mixture is recrystallized in the beaker. Then the recrystallized solid was taken out and placed in a ball mill with a rotation speed of 600rpm-800rpm and a number of turns of 2 to obtain a uniform catalyst precursor of urea, glucose, cobalt chloride hexahydrate and sodium thiocyanate.
[0055] (2) Preparation of single-atom cobalt-based nitrogen-sulfur double-doped carbon catalyst (CoNSG3)
[0056] First, place the above-prepared precursor i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com