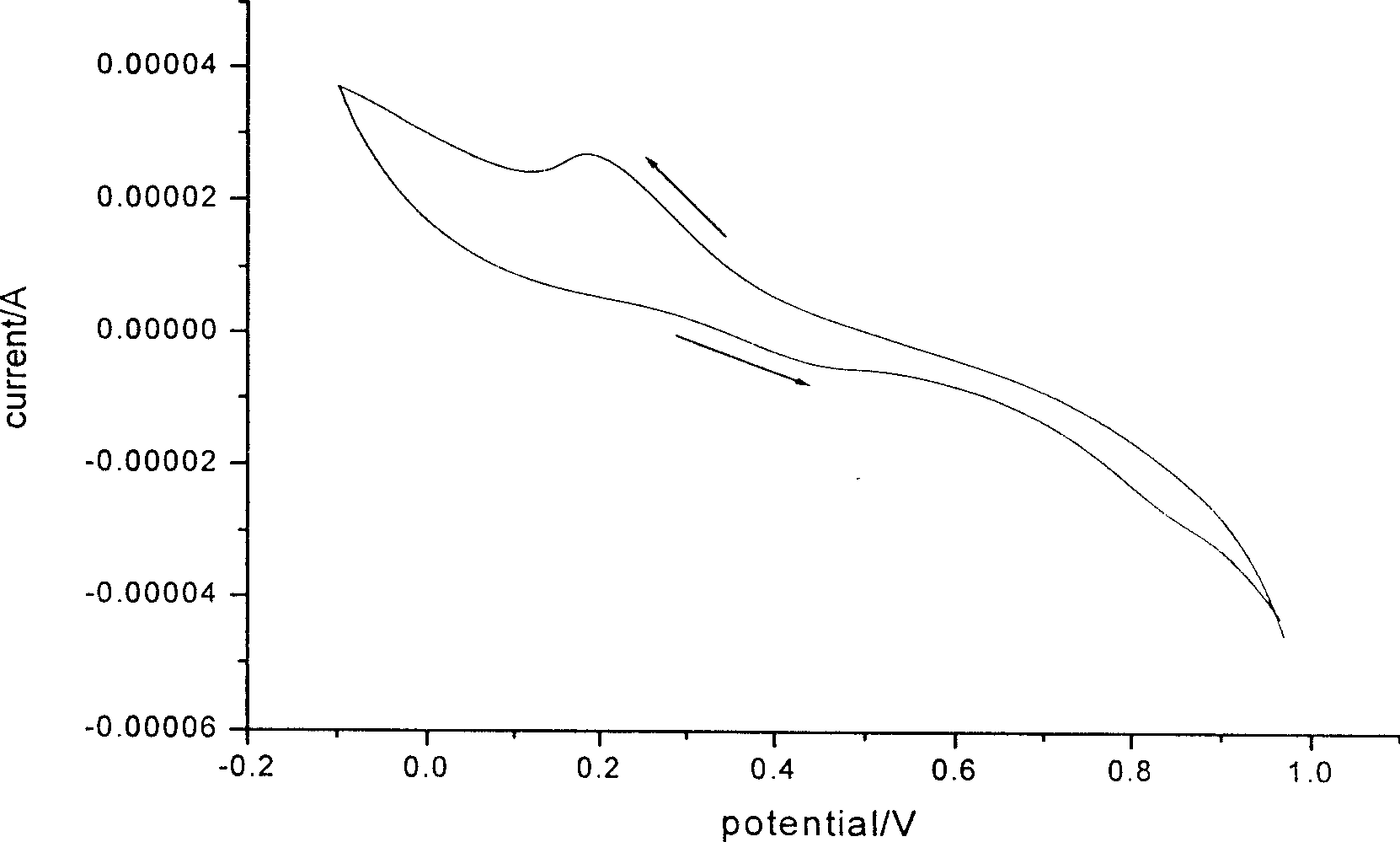
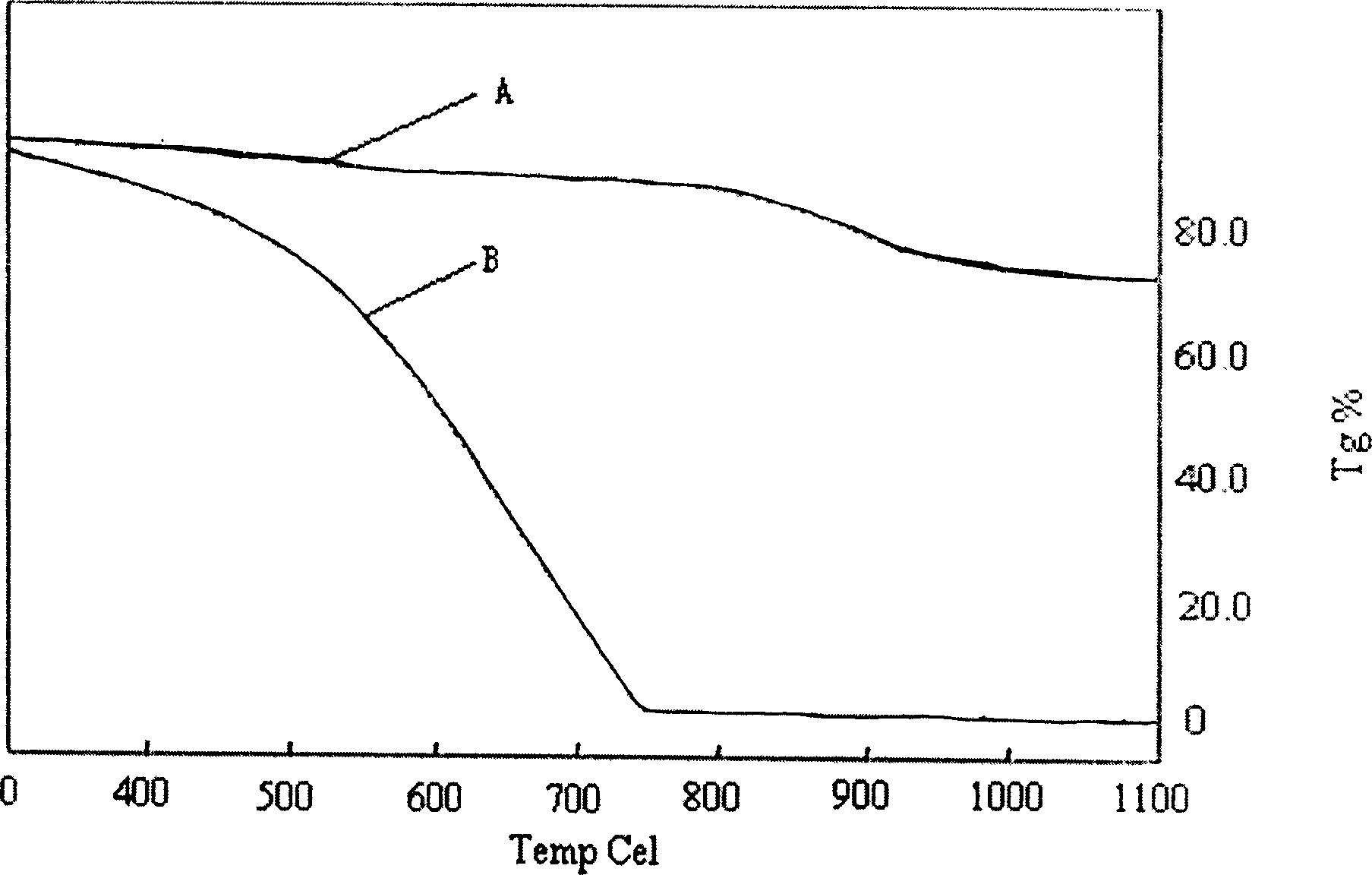
Water-soluble high temperature-resistant polyaniline conducting material and its preparation method
A technology of polyaniline and conductive materials, applied in the direction of non-metallic conductors, organic material conductors, etc., can solve the problems of not having high temperature resistance, and achieve the effects of reducing the amount of enzymes, reducing costs, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Weigh 0.108 g of p-phenylenediamine, fully dissolve it in 8 ml of deionized water, add 0.071 g of acrylic acid, stir and mix evenly; completely dissolve 2 mg of horseradish peroxidase in disodium hydrogen phosphate and dihydrogen phosphate at pH=7 Sodium mixed buffer solution, and then added to the above-mentioned mixed solution; after fully mixed uniformly, drop into the weight ratio of 0.3% H 2 O 2 The solution, 0.4 mg each time, add 1 time every 20 minutes for a total of 6 times; after the hydrogen peroxide is added, continue to react for 20 hours, under nitrogen protection; then, the product and the reaction solution are added to the acetone solution for precipitation, A black powdery substance is obtained; washed with acetone several times to remove unreacted monomers and part of low molecular weight polymers; vacuum dried at 48°C for 52 hours to obtain the product of the present invention.
Embodiment 2
[0025] Weigh 0.108 g of p-phenylenediamine, fully dissolve it in 8 ml of deionized water, add 0.036 g of acrylic acid, stir and mix evenly; completely dissolve 120 mg of horseradish peroxidase in disodium hydrogen phosphate and dihydrogen phosphate at pH=7 Sodium mixed buffer solution, and then added to the above-mentioned mixed solution; after fully mixed uniformly, drop into 0.03% H by weight 2 O 2 Solution, 2 mg each time, add 1 time every 35 minutes for a total of 4 times; after the hydrogen peroxide is added, continue to react for 30 hours, under nitrogen protection; then, the product and the reaction solution are added to the acetone solution for precipitation, The black powdery substance is obtained, and then washed with acetone for many times to remove unreacted monomers and part of low molecular weight polymers; vacuum drying at 80°C for 45 hours to obtain the product of the present invention.
Embodiment 3
[0027] Weigh 0.108 g of p-phenylenediamine, fully dissolve it in 8 ml of deionized water, add 0.018 g of acrylic acid, stir and mix well. 60 mg of horseradish peroxidase was completely dissolved in a buffer solution of pH=7 mixed of disodium hydrogen phosphate and sodium dihydrogen phosphate, and then added to the above-mentioned mixed solution. After mixing well, add 0.15% H by weight 2 O 2 Solution, 1 mg each time, add 1 time every 30 minutes for a total of 5 times. After the hydrogen peroxide was added, the reaction was continued for 25 hours, and it was filled with nitrogen for protection. Then, the product and the reaction liquid are added to the acetone solution for precipitation to obtain a black powdery substance, which is then washed with acetone several times to remove unreacted monomers and some low molecular weight polymers. Vacuum drying at 60°C for 50 hours to obtain the product of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com