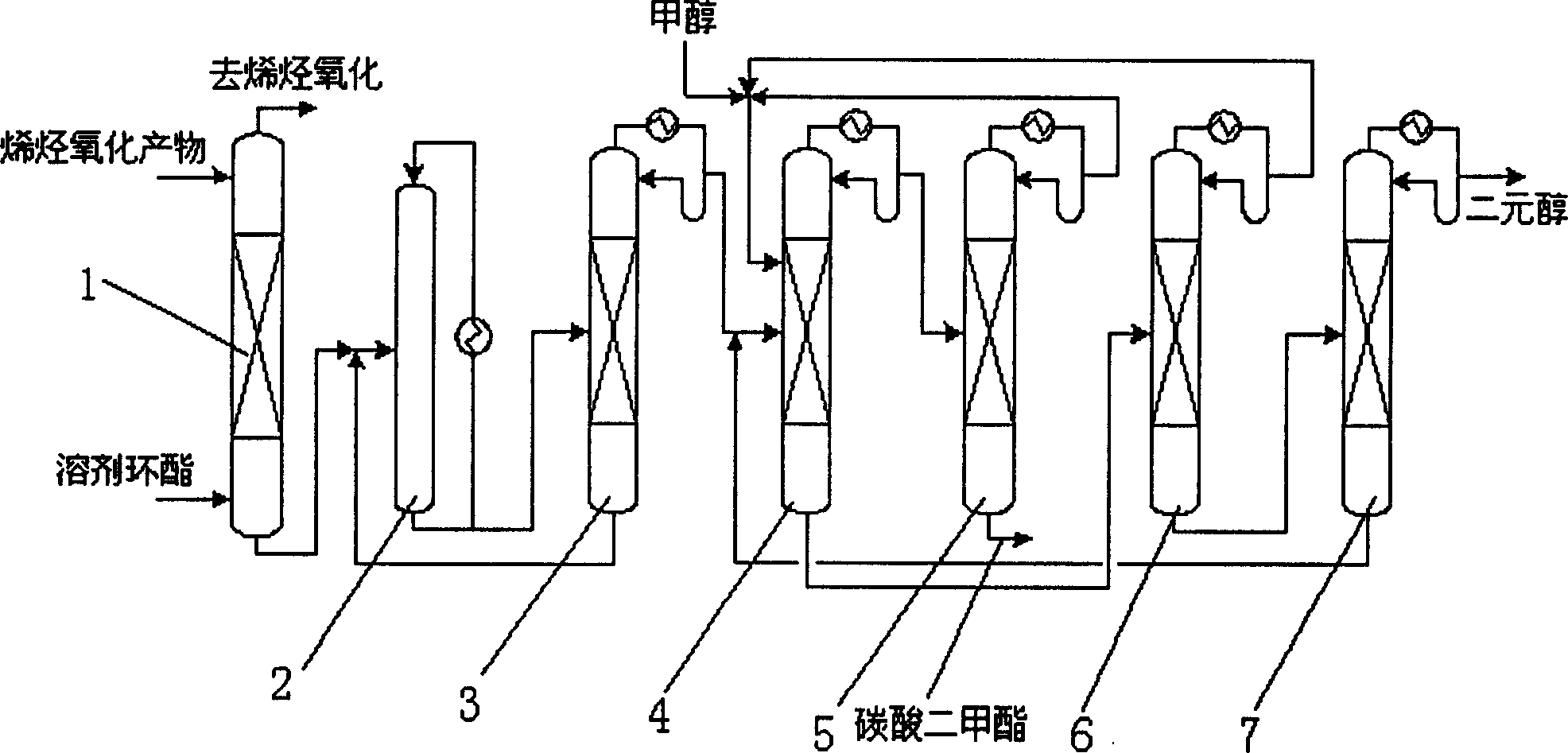
Method of distillation and ester exchange reaction for producing dimethyl carbonate and dihydroxyl alcohols
A technology of dimethyl carbonate and reactive distillation, which is applied in the preparation of organic carbonates and organic chemistry, can solve the problems of single-pass conversion rate limitation and high requirements for the pretreatment of reaction raw materials, and achieve the goal of reducing energy consumption and increasing single-pass conversion rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Catalyzed esterification reaction of ethylene oxide and carbon dioxide to generate ethylene carbonate
[0032] The gas obtained from the oxidation of ethylene contains 1.5 mol% of ethylene oxide and 7.5 mol% of carbon dioxide. The temperature is 45°C. The flow rate of 100kg / h passes through a packed tower with a bed height of 1.2 meters. The ethylene carbonate absorbing liquid at 45°C It flows down from the top of the tower at a flow rate of 75kg / h, contacts with the gas countercurrently, and operates at a pressure of 1.8MPa. Ethylene oxide and carbon dioxide are absorbed into the absorption liquid. Analysis shows that the absorption rate of ethylene oxide is over 99.5%, and the absorption rate of carbon dioxide is over 90%.
[0033] Mix the above absorbing liquid with the catalyst tetraethylammonium bromide in a reactor, and heat it to 150°C, and the operating pressure is 8MPa. After 45 minutes of reaction, the conversion rate of ethylene oxide is above 99%, and no ...
Embodiment 2
[0044] 1. Catalyzed esterification reaction of propylene oxide and carbon dioxide to generate propylene carbonate
[0045] Containing 1.5mol% of propylene oxide and 7.5mol% of carbon dioxide in the gas obtained from the oxidation of propylene, the temperature is 35°C, and the flow rate of 100kg / h passes through a packed tower with a bed height of 1.2 meters, and the propylene carbonate absorption solution at 35°C is The flow rate of 75kg / h flows down from the top of the tower and contacts with the gas countercurrently, the operating pressure is 1.6MPa, and the propylene oxide and carbon dioxide are absorbed into the absorption liquid. Analysis shows that the absorption rate of propylene oxide is more than 99.5%, and the absorption rate of carbon dioxide is more than 90%.
[0046] Mix the above absorbing liquid with the catalyst tetrapropylammonium bromide in the reactor, and heat it to 150°C, the operating pressure is 8MPa, after 45 minutes of reaction, the conversion rate of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com