Process for producing gene engineering immobilized enzyme N-glycoamidase
A technology of sugar amidase and immobilized enzyme, which is applied to other methods of inserting foreign genetic materials, recombinant DNA technology, DNA/RNA fragments, etc., can solve the problems that limit the widespread use of N-glycoamidase and large-scale preparation, and achieve The production method is simple and easy, the stability is high, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Construction of recombinant expression vector:
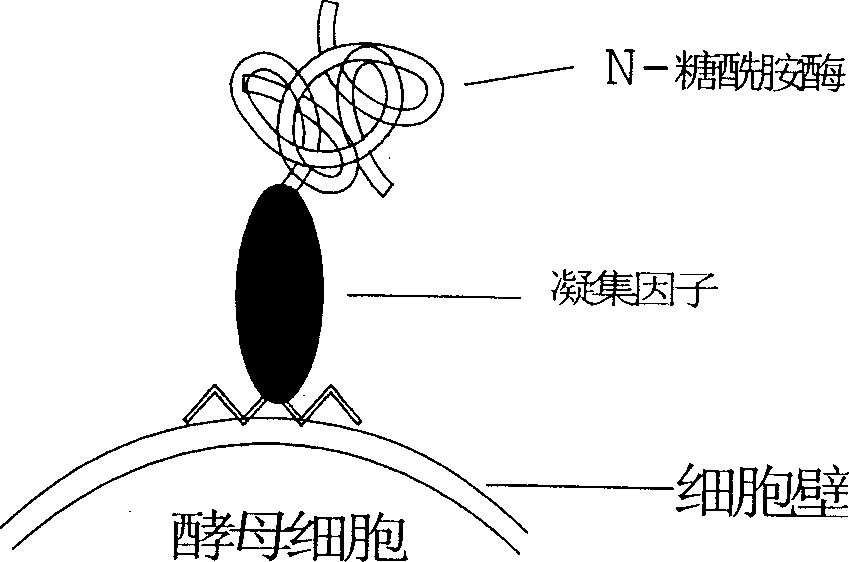
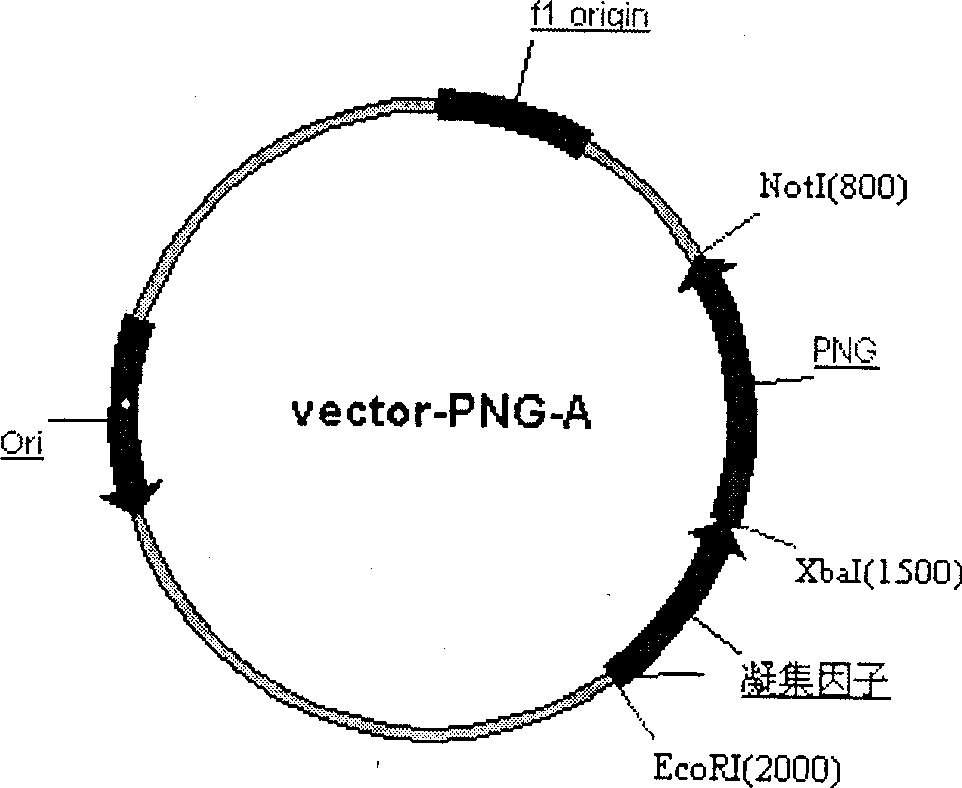
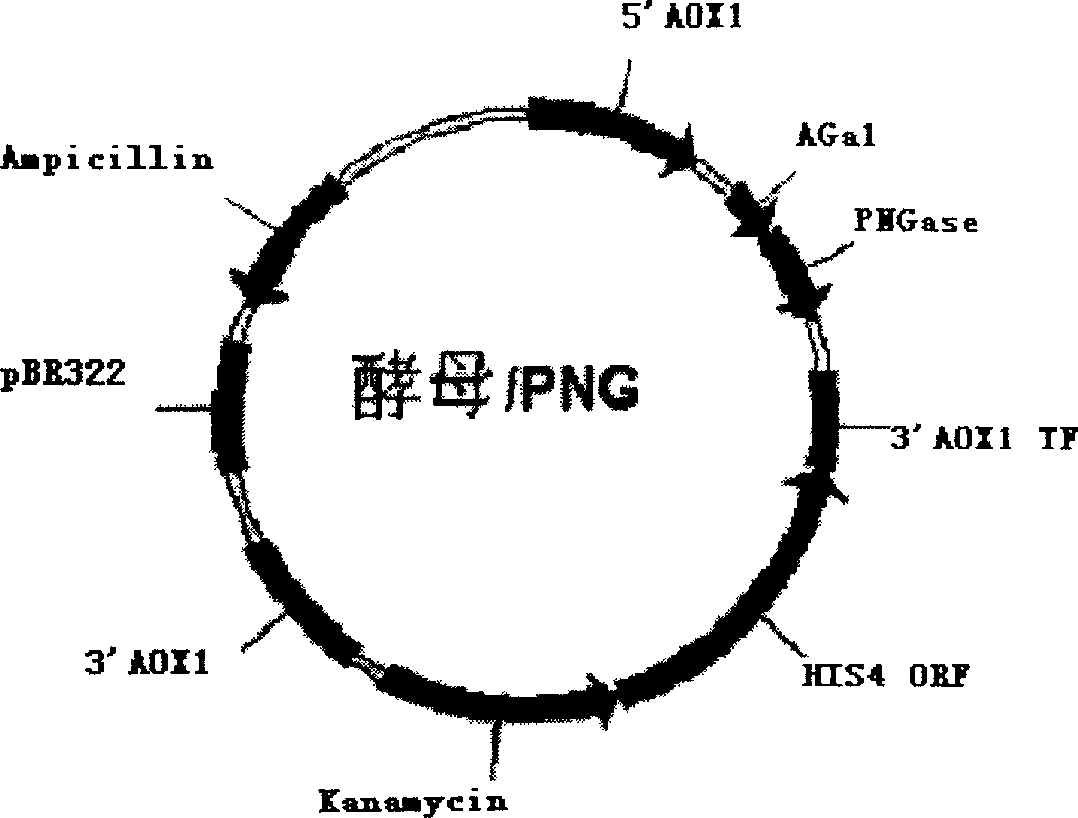
[0042] Using 5-ATATGAATTCGCTCCGCCAGATAATACCGT-3; 5-CACGTCTAGAGTTTGTAACTACCGGAG-3 as primers to clone the N-glycoamidase gene from Pseudomonas meningitidis ATCC 33958 (purchased from ATCC), PCR amplification conditions: 50 μl reaction system, 10×PCR buffer 5 μl , MgCl 2 (25mmol / L) 3μl, d NTP (25mmol / L) 1μl, primers 1 and 2 (20pmol / L) 1μl each, genomic DNA 1μl, Taq DNA polymerase 0.5U, pre-denaturation at 94°C for 5min, according to the following parameters: Denaturation at 94°C for 45 s, annealing at 56°C for 45 s, extension at 72°C for 2 min, 28 cycles, and the last cycle of extension at 72°C for 10 min. The XbaI and NotI sites inserted into the pBluescriptSK(+) vector after digestion with XbaI and NotI were named vector-PNG. Then use 5-TGCATCTAGACGCCAAAAGCTCTTTTATCT-3; 5-TCAGCGGCCGCTTTGATTATGTTCTTTCT-3 as primers to clone the Saccharomyces cerevisiae coagulation factor C-terminal anchor site gene, PCR amplification...
Embodiment 2
[0054] (1) Construction of recombinant expression vector:
[0055] Using 5-ATATGAATTCGCTCCGCCAGATAATACCGT-3; 5-CACGTCTAGAGTTTGTAACTACCGGAG-3 as primers to clone the N-glycoamidase gene from Pseudomonas meningitidis ATCC 33958 (purchased from ATCC), PCR amplification conditions: 50 μl reaction system, 10×PCR buffer 5 μl , MgCl 2 (25mmol / L) 3μl, d NTP (25mmol / L) 1μl, primers 1 and 2 (20pmol / L) 1μl each, genomic DNA 1μl, Taq DNA polymerase 0.5U, pre-denaturation at 94°C for 5min, according to the following parameters: Denaturation at 94°C for 45 s, annealing at 56°C for 45 s, extension at 72°C for 2 min, 28 cycles, and the last cycle of extension at 72°C for 10 min. The XbaI and NotI sites inserted into the pBluescriptSK(-) vector after digestion with XbaI and NotI were named vector-PNG. Then use 5-TGCATCTAGACGCCAAAAGCTCTTTTATCT-3; 5-TCAGCGGCCGCTTTGATTATGTTCTTTCT-3 as primers to clone the Saccharomyces cerevisiae coagulation factor C-terminal anchor site gene, PCR amplification...
Embodiment 3
[0067] (1) Construction of recombinant expression vector:
[0068] Using 5-ATATGAATTCGCTCCGCCAGATAATACCGT-3; 5-CACGTCTAGAGTTTGTAACTACCGGAG-3 as primers to clone the N-glycoamidase gene from Pseudomonas meningitidis ATCC 33958 (purchased from ATCC), PCR amplification conditions: 50 μl reaction system, 10×PCR buffer 5 μl , MgCl2 (25mmol / L) 3μl, d NTP (25mmol / L) 1μl, primers 1 and 2 (20pmol / L) 1μl each, genomic DNA 1μl, Taq DNA polymerase 0.5U, pre-denaturation at 94°C for 5min, according to the following parameters: Denaturation at 94°C for 45 s, annealing at 56°C for 45 s, extension at 72°C for 2 min, 28 cycles, and the last cycle of extension at 72°C for 10 min. The XbaI and NotI inserted into the pET22b vector after digestion with XbaI and NotI were named vector PNG. Then use 5-TGCATCTAGACGCCAAAAGCTCTTTTATCT-3; 5-TCAGCGGCCGCTTTGATTATGTTCTTTCT-3 as primers to clone the Saccharomyces cerevisiae coagulation factor C-terminal anchor site gene, PCR amplification conditions are th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com