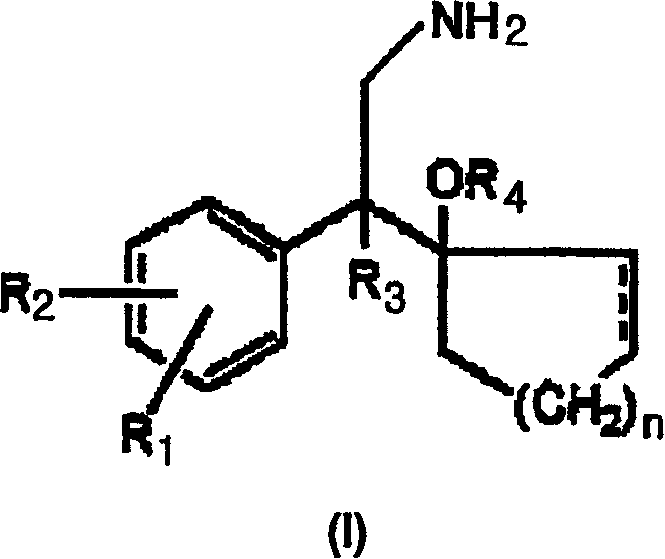
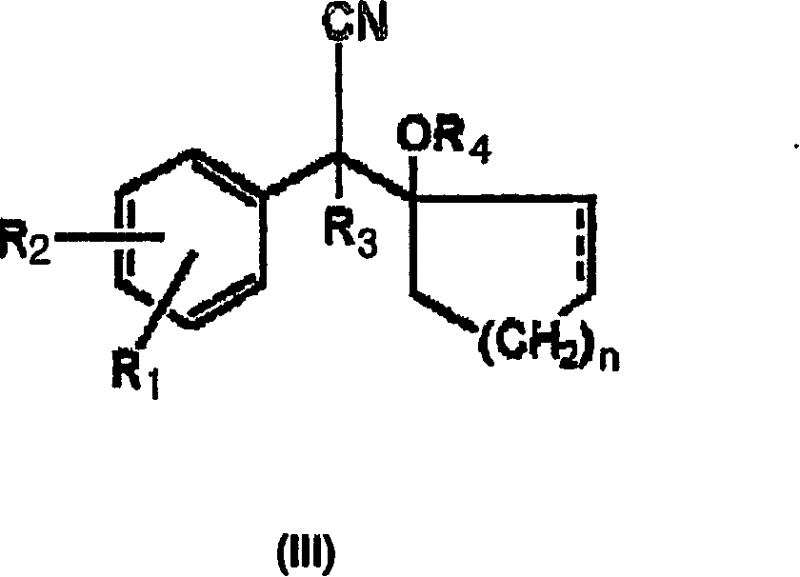
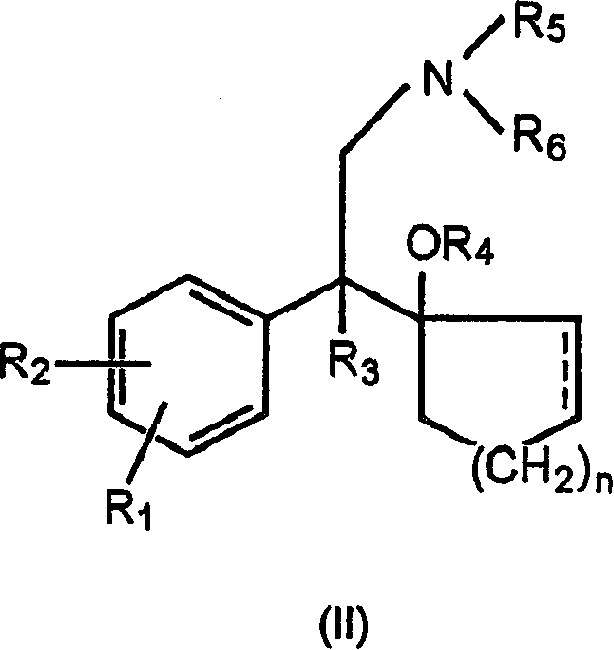
Process for preparation of phenethylamine derivatives
A technology for compounds and composites, applied in the field of preparing phenethylamine derivatives, can solve problems such as being difficult to separate final products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 25.0 g of Raney nickel (Kawaken, Grace 2400, 2724, Degussa B111W, 112W) and 250 ml of methanol and 25 ml of ammonia solution (25% NH 3 ). To the mixture was added 50 g of 1-[cyano(4-methoxyphenyl)methyl]cyclohexanol, followed by 250 ml of MeOH. Fix the reactor and fill it with N 2 Gas purge two or three times. In use N 2 After thorough cleaning, use H 2 Gas purges the reactor two or three times. The internal pressure of the reactor was adjusted to 60 psi and the reaction mixture was stirred. After 20 to 30 hours of reaction at room temperature (approximately 10 to 20° C.), an in-process analysis of a sample of the mixture was performed. After the reaction was complete the Raney nickel was removed by filtration through a pad of celite. The filtrate was distilled under reduced pressure until all the solvent was evaporated, and 200 ml of isopropanol and 400 ml of ethyl acetate were added to the residual oil to dissolve the resulting product, resulting in a clear or ...
Embodiment 2 to 10
[0047] Tests were performed in the same manner as in Example 1, except that the amounts of raw materials, Raney nickel, and ammonia solution; reaction temperature; and reaction time were shown in the table below. The purity and yield of the desired product (r.t. = room temperature from about 10 to 20° C.) is also indicated in the last column of the table.
[0048] Example
Embodiment 11
[0050] Add 40ml of H into a graduated cylinder (mass cylinder) 2 After O, place the graduated cylinder on the balance. Then return the balance to zero. Add Raney nickel to the vector cylinder and remove the increased volume of water, maintaining a total volume of 40ml, until the balance shows a total weight of 12g. Remove H 2 O and add 40g fresh H 2 O, to wash the catalyst. After washing, the removal of H 2 O. Add Raney nickel to the reactor, while using 20ml of H 2 O and 600 MeOH rinse. To the mixture was added 40 g of 1-[cyano(4-methoxyphenyl)methyl]cyclohexanol, followed by 20 ml of ammonia solution. The reactor was fixed and then filled with N 2 Gas purge two or three times. In use N 2 After adequate purging, the reactor was purged two or three times with hydrogen. The internal pressure of the reactor was adjusted to 60 psi, and stirring was started. After 20 to 30 hours of reaction at room temperature, an in-process analysis of a sample of the mixture was per...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com