High purity metaphosphate and method for production thereof
A technology for metaphosphates and manufacturing methods, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve problems such as difficult molar ratio control, detailed manufacturing methods, and no records of impurities and no impurity content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0090] (1) The first process
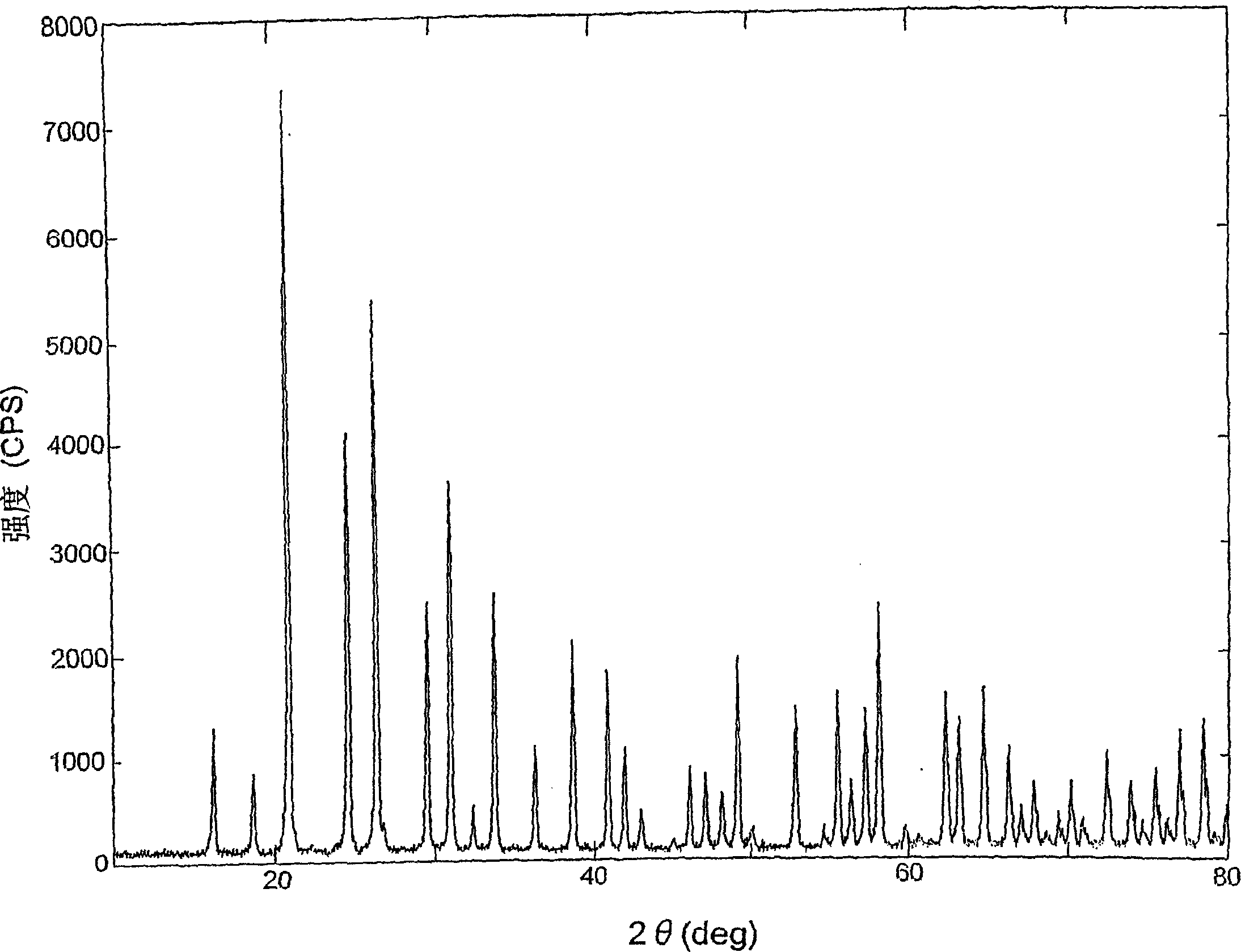
[0091] Phosphoric acid (manufactured by Nippon Chemical Industry Co., Ltd., H 3 PO 4 Concentration 85%, pure phosphoric acid) 345.9g add in the beaker of 2 liters, add high-purity aluminum hydroxide 78.0g again. Convert to P 2 o 5 and Al 2 o 3 The molar ratio (P 2 o 5 / Al 2 o 3 ) is 3.00. Heat the beaker with an electric heater to start the reaction. The heat of reaction raises the temperature of the liquid to around 120°C. Stay in this state for 30 minutes. The aluminum dihydrogen phosphate reaction solution is generated through the reaction.
[0092] (2) Second process
[0093] The aluminum dihydrogen phosphate reaction solution obtained in the first process is transferred to a sintered container made of aluminum that has previously been laid with aluminum metaphosphate powder. The sintered container was put into an electric furnace and heated to 550°C, and kept at this temperature for 4 hours for sintering. After the sintering ...
Embodiment 1-2
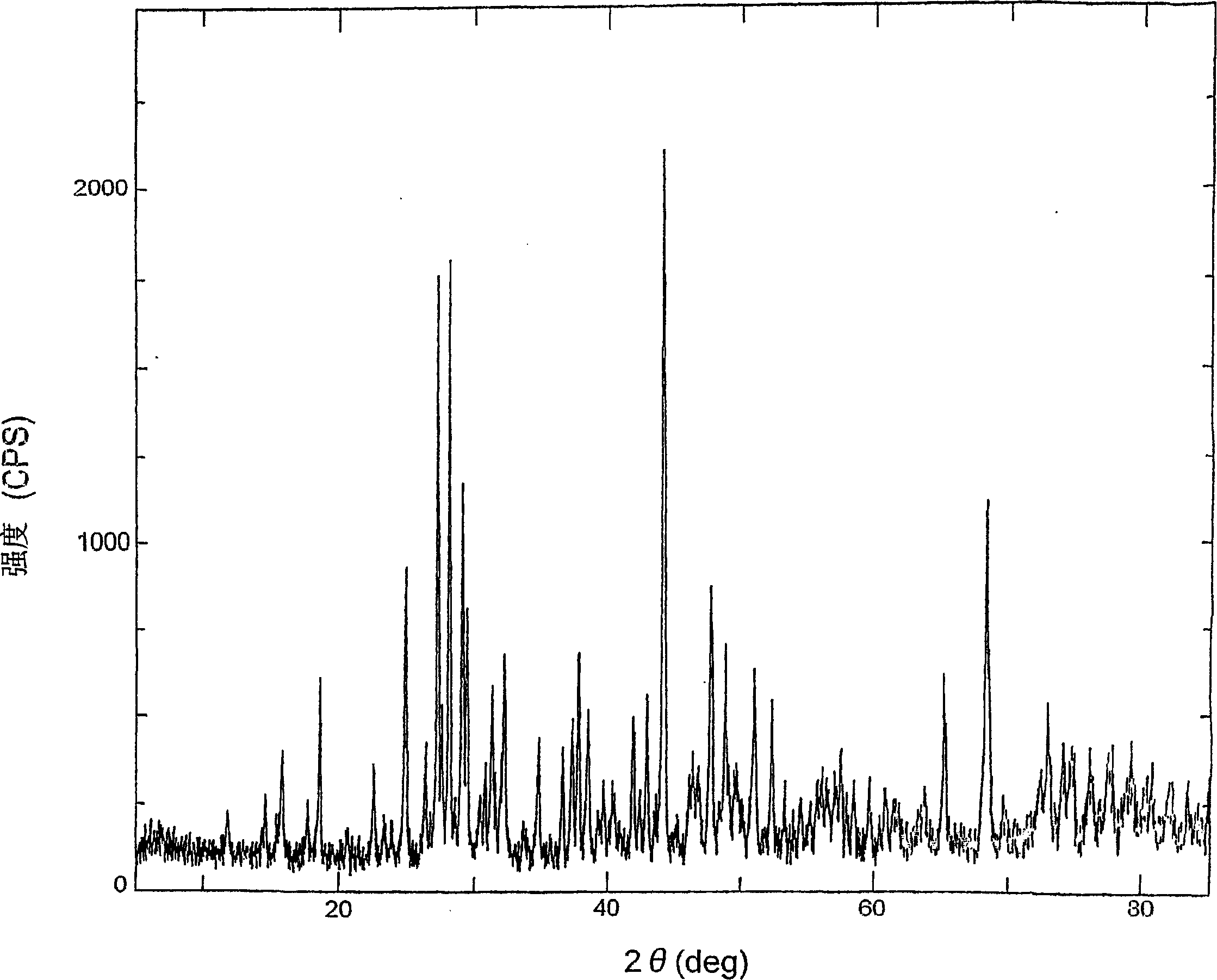
[0097] Wash the aluminum metaphosphate powder obtained in the third process in Example 1 with pure water, and dry it with a drier. Others are the same as in Example 1-1.
Embodiment 1-3
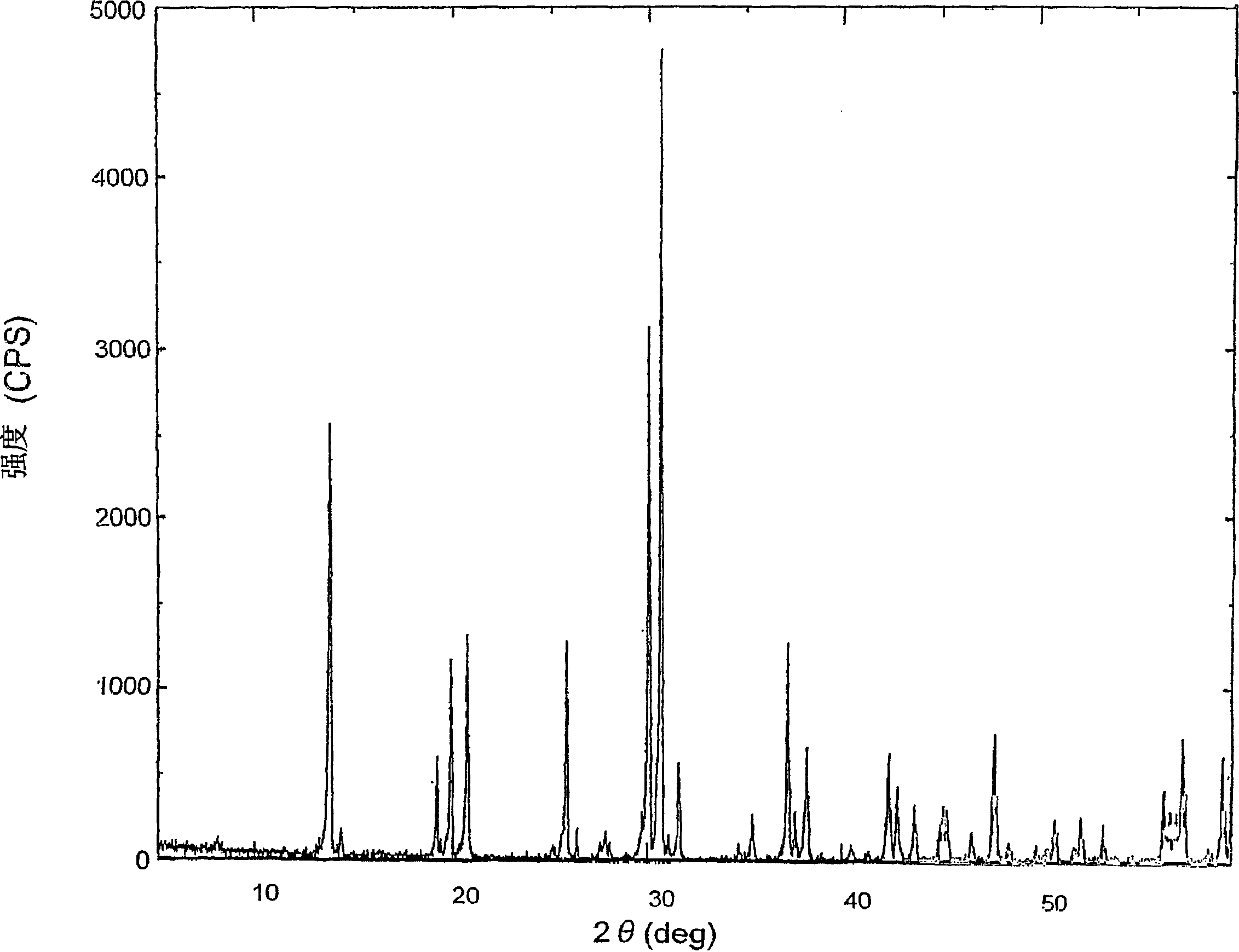
[0099] Phosphoric acid (manufactured by Nippon Chemical Industry Co., Ltd., H 3 PO 4 Concentration: 85% by weight, 345.9 g of pure phosphoric acid) and 27 g of pure water were put into a 2-liter beaker, and then 51 g of α-alumina was added. Convert to P 2 o 5 and Al 2 o 3 The molar ratio (P 2 o 5 / Al 2 o 3 ) is 3.00:1. Heat the beaker with an electric heater to start the reaction. The heat of reaction raises the temperature of the liquid to around 130°C. Stay in this state for 30 minutes. The aluminum dihydrogen phosphate reaction solution is generated through the reaction. Thereafter, the second process and the third process in Example 1-1 were carried out to obtain aluminum metaphosphate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com