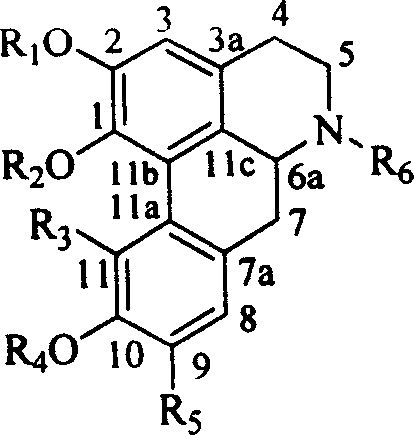
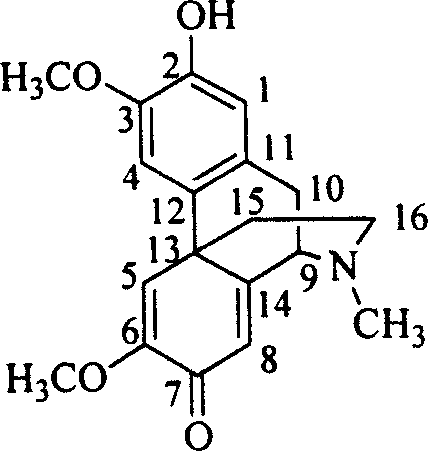
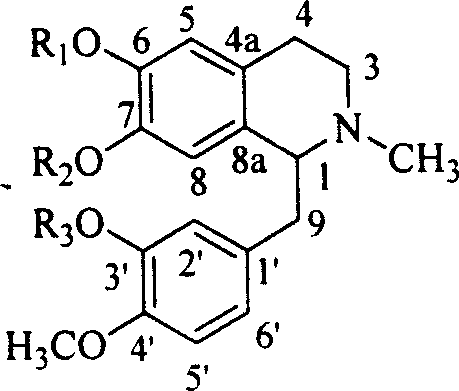
Lindera root alkaloid, its preparation method and application in medicine preparation
A kind of technology of alkaloid and black medicine, which is applied in the direction of drug combination, medical formula, antipyretic, etc., can solve the problems of damage to gastrointestinal mucosa, poor action selectivity, induction of infection, etc., to alleviate rheumatic diseases, reduce content, The effect of reducing swelling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0031] The preparation of example 1, black medicine alkaloid
[0032] Method: Weigh 2 kg of black herb powder, heat and reflux with 90% ethanol to extract 3 times, the amount of 90% ethanol is 12000mL each time, extract for 2 hours each time, combine the extracts, filter, concentrate to dryness under reduced pressure, add 2 2000mL of % HCl solution, dissolved in alkaloids, filtered, and the filtrate was adjusted to pH 8.5 with ammonia water, stirred thoroughly, and left to stand for 5 hours. After the precipitate was fully analyzed, the precipitate was filtered out, and dried under reduced pressure at 55°C to obtain the black medicine Alkali is a light brown powder. The weighed weight is 32g. The yield is 1.60%. After determination, the content of total alkaloids is 53.13%, and the content of norisoboldine is 33.84%.
example 2
[0033] Example 2, the influence of alkaloids of the present invention on mouse arthritis caused by type II collagen
[0034] Chicken type II collagen and Freund's complete adjuvant were fully ground and emulsified in a cold water bath to prepare 1.5mg / ml. Male ICR mice were intradermally injected with 100 μl of the above-mentioned emulsion at the base of the tail. The same operation was taken on the 21st day, and the mice were grouped by body weight. The administration group was fed with 50, 100, and 200 mg / kg of alkaloids of the present invention, and the positive control group was fed with dexamethasone (dexamethasone acetate sheet, Zhejiang Xianju Pharmaceutical Co., Ltd. product, batch number 040902) 1mg / kg and tripterygium glycosides (tripterygium glycosides tablets, product of Shanghai Fudan Fuhua Pharmaceutical Co., Ltd., batch number 020601) 15mg / kg. The control group and the normal group were intragastrically administered equal volume of distilled water, once a day, ...
example 3
[0045] Example 3, the effect of in vitro administration of alkaloids of the present invention on the release of nitric oxide from LPS-induced mouse peritoneal macrophages
[0046] Male ICR mice were sacrificed by decapitation and bloodletting, and peritoneal macrophages were collected under sterile conditions, and the cell concentration was adjusted to 2×10 6 pieces / ml. Add the cells to a 96-well plate, the number of cells per well is 2×10 5 , adhere to the wall for 2 hours, wash twice with RPMI-1640. Add LPS (final concentration of 10 μg / ml) and different concentrations of drugs (dissolved in RPMI-1640 and DMSO, final concentration of DMSO is 0.5%) to each well, incubate for 24 hours, absorb 100 μl of supernatant, and react with the same amount of Griess reagent for 10 Minutes, the OD value was measured at 540nm.
[0047] It can be seen from Table 5 that 50 and 100 μg / ml of alkaloids of the present invention significantly inhibited the release of NO from mouse peritoneal m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com