Pgk1 protein, recombinant plasmid for expressing Pgk1 protein, recombinant probiotic for expressing Pgk1 protein and application
A technology of recombinant plasmids and probiotics, applied in the direction of peptide/protein components, applications, recombinant DNA technology, etc., to improve neurological behavior, significant therapeutic effect, and reduce the volume of cerebral infarction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
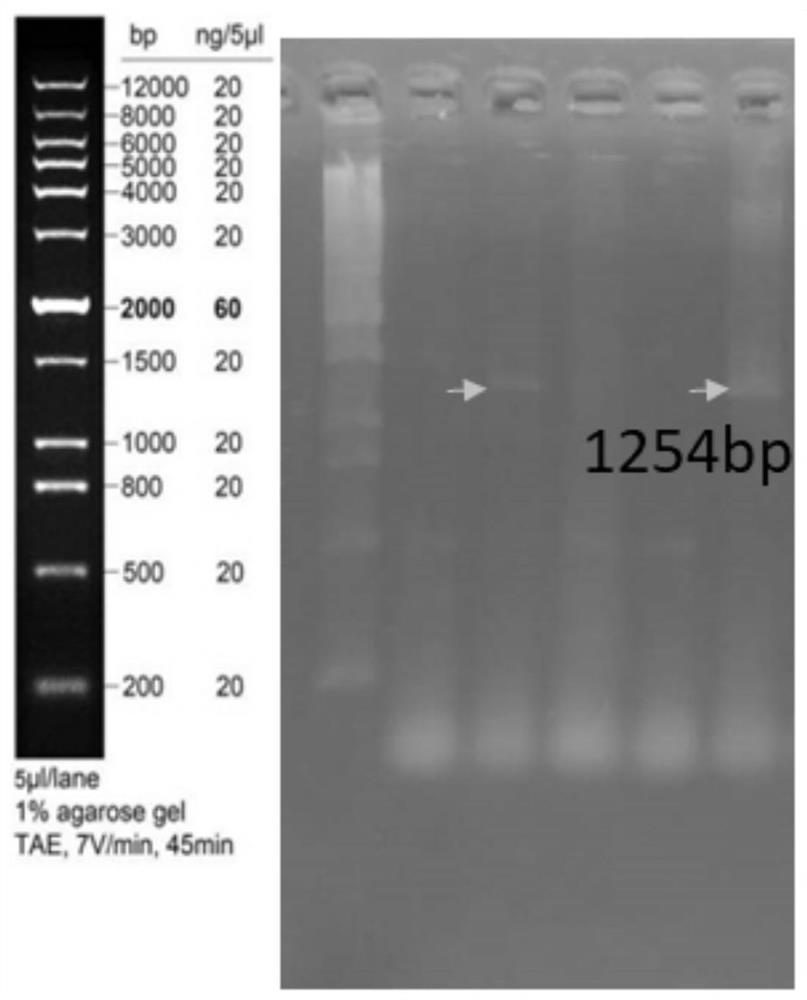
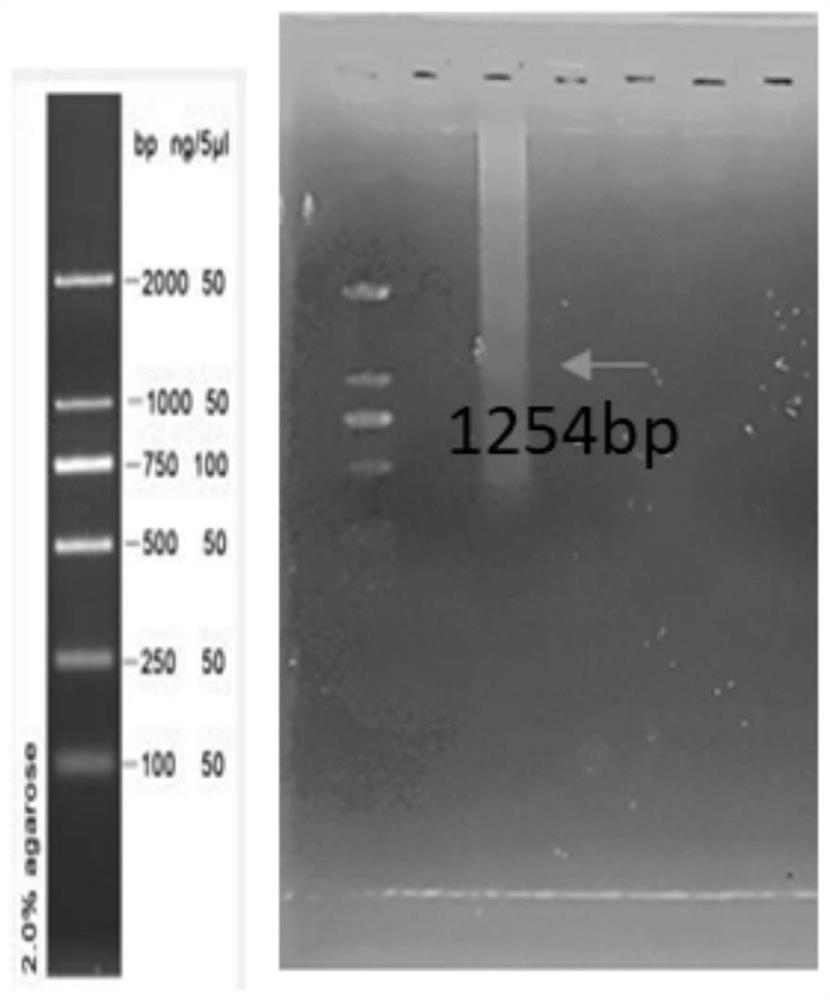
[0059] Example 1 Preparation of recombinant plasmids expressing Pgk1 and recombinant probiotics expressing Pgk1
[0060] 1. Primer Synthesis
[0061] According to the nucleotide sequence of the gene Pgk1 (shown in SEQ ID NO.2), compare it with the pET-28a plasmid map, design specific primers, and insert restriction endonuclease sites BamHI and EcoRI into the primers, specific information See Table-1. The primers were synthesized by Shanghai Sangon Bioengineering Co., Ltd.; the primers were dissolved in sterile deionized water to make a concentration of 10 μmol / L.
[0062] Table 1 Primer information and synthesis
[0063]
[0064] 2. Construction of recombinant plasmids
[0065] The pET-28a-Pgk1 plasmid was synthesized by Jinweizhi Biotechnology Co., Ltd.
[0066] 3. Transform Escherichia coli DH5α bacteria with recombinant plasmid
[0067] Take out the DH5α competent cells from the -80℃ refrigerator, and place them on the ice box for 10-20 minutes to melt them; take out...
Embodiment 2
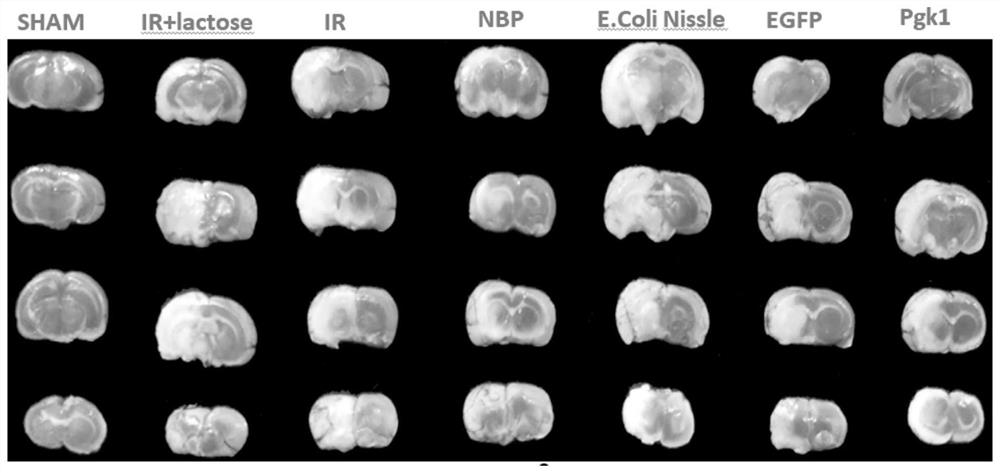
[0079] Example 2 Therapeutic Effect of Recombinant Probiotics Expressing Pgk1 on Ischemic Stroke Rats
[0080] 1. Preparation and administration time of MCAO model:
[0081] SPF grade healthy male SD rats, weighing 180-220g, were randomly divided into 7 groups after one week of adaptive feeding, and the grouping and dosage were as follows:
[0082] Sham operation group (SHAM, orally administered β-lactose solution of equal volume 11.1mg / ml);
[0083] MCAO ischemia-reperfusion model group (IR, orally administered an equal volume of 0.9% normal saline);
[0084] MCAO model+lactose group (IR+lactose, oral administration of equal volume of 11.1mg / ml β-lactose solution);
[0085] MCAO model + positive drug group (NBP, oral administration of butylphthalide 20mg / kg / day);
[0086] MCAO model + E.coli Nissle 1917 group: Escherichia coli (Escherichia coli) Nissle 1917 original bacteria of 2.5mL orally administered;
[0087] MCAO model+pET-28a-EGFP group: intragastric administration ...
Embodiment 3
[0102] Example 3 The therapeutic effect of recombinant probiotics expressing Pgk1 on diabetic mice
[0103] 1. Establishment of C57BL / 6N diabetic mouse model
[0104] SPF grade healthy male C57BL / 6N mice, weighing 18-22g.
[0105] Sixty C57BL / 6N mice were randomly divided into three groups:
[0106] Common diet group: SD group, 8 C57BL / 6N mice. After feeding with common feed for three weeks, intraperitoneally inject citrate buffer solution (the injection dose is the same as the following STZ solution), fasting and water for 12 hours before injecting citrate buffer solution, inject according to the same time period 9:00am-10:00am, Inject once a day for five consecutive days, and feed after 2 hours of citric acid buffer solution injection. Then feed with common feed for another three weeks, fasting without food and water for 10 hours, and detect fasting blood glucose of mice between 7:30pm-9:00pm.
[0107]Type 2 diabetes model group: high-fat diet + streptozotocin group, HFD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com