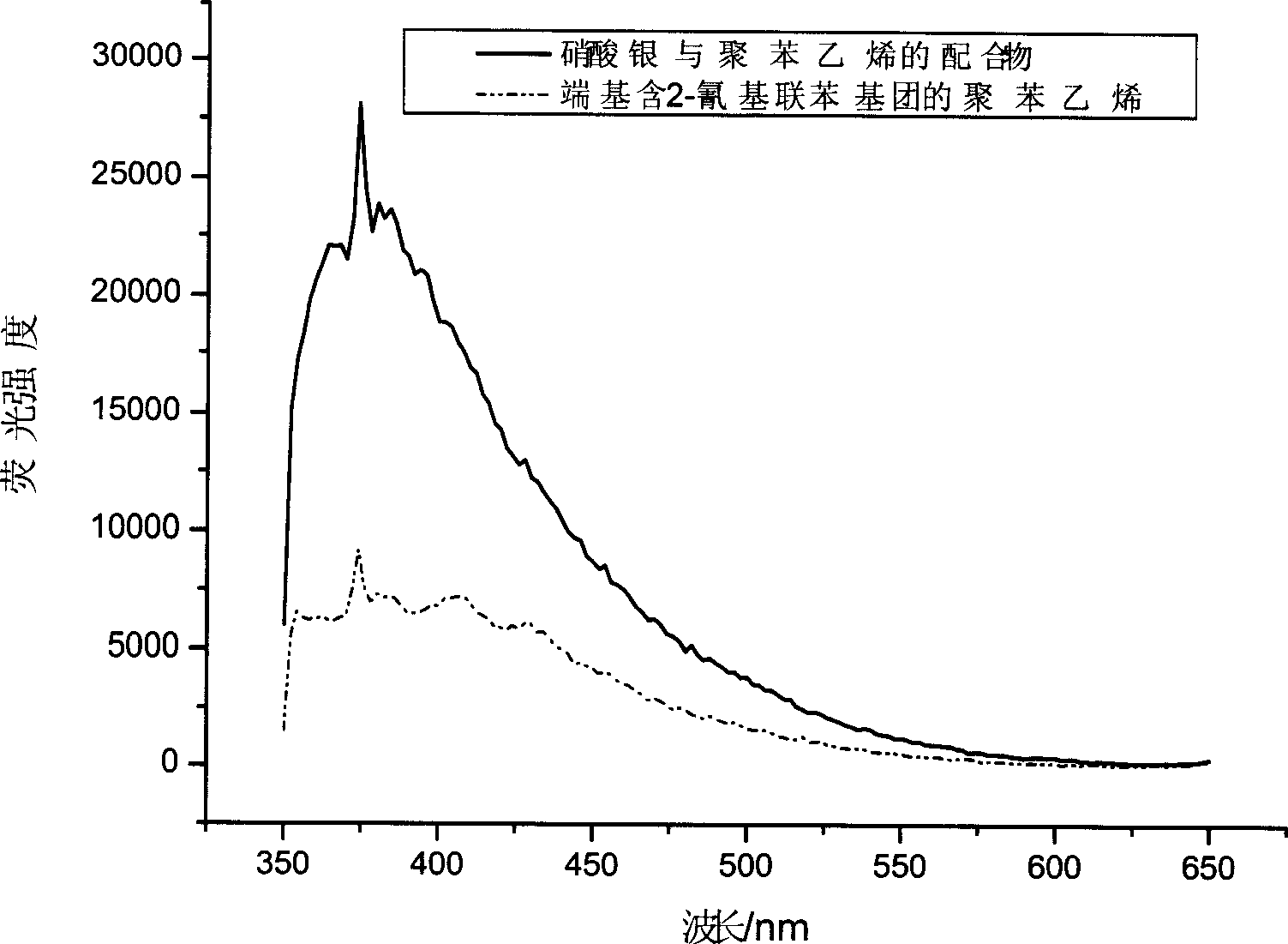
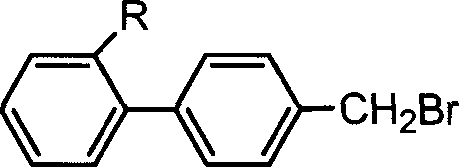
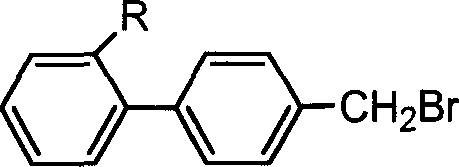
Uses of 4-bromomethylbiphenyl and 2-substituted 4'-bromomethylbiphenyl
A technology of bromomethyl biphenyl and biphenyl, which is applied in the field of biphenyl compounds, can solve problems such as no initiators, and achieve the effects of good fluorescence performance, enhanced fluorescence performance and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of polymers containing biphenyl groups at the end groups
[0026] The initiator 4-bromomethylbiphenyl (the molar ratio to the monomer is 1:25~1:500), the catalyst [pentamethyldiethyltriamine and cuprous bromide (or cuprous chloride) complex, the molar ratio of the two to the initiator is (0.5~1): 1:1], the monomer (styrene and methyl methacrylate) is added to the solvent (cyclohexanone, and the volume ratio of the monomer is 0 : 1 to 5: 1), seal the reaction vessel, then evacuate and fill with nitrogen, and carry out the polymerization reaction at the set temperature (within the range of 20 to 100° C.) in the reaction vessel after deoxygenation. To the set time (depending on the polymer molecular weight, monomer and temperature to be controlled, the time can range from 0.5 to 24 hours), take it out and add tetrahydrofuran to dilute, and then pour it into excess methanol with 1:1 hydrochloric acid Precipitate, filter. The polymer is dissolved in ...
Embodiment 2
[0027] Embodiment two: the preparation of the polymer that end group contains 2-cyanobiphenyl
[0028] The initiator 2-cyano-4'-bromomethylbiphenyl (the molar ratio to the monomer is 1:25~1:500), the catalyst [pentamethyldiethyltriamine and cuprous bromide (or Cuprous chloride) complexes, the molar ratio between the two and the initiator is (0.5~1): 1:1], monomers (styrene and methyl methacrylate) are added to the solvent cyclohexanone (with mono volume ratio of 0:1 to 5:1), the reaction vessel was sealed, and then repeatedly evacuated and filled with nitrogen. The reaction vessel after deoxygenation is subjected to polymerization reaction at a set temperature (from room temperature in the range of 20 to 100° C.). To the set time (depending on the polymer molecular weight, monomer and temperature to be controlled, the time can range from 0.5 to 24 hours), take it out and add tetrahydrofuran to dilute, and then pour it into excess methanol with 1:1 hydrochloric acid Precipita...
Embodiment 3
[0030] Embodiment three: the preparation of the polymer that terminal group contains 2-carboxylate biphenyl
[0031] The initiator 4'-bromomethylbiphenyl-2-carboxylate (the molar ratio to the monomer is 1:25~1:500), the catalyst [pentamethyldiethyltriamine and cuprous bromide ( or cuprous chloride), the molar ratio of the two to the initiator is (0.5~1): 1:1], the monomer (styrene and methyl methacrylate) is added to the solvent cyclohexanone (with In a monomer volume ratio of 0:1 to 5:1), the reaction vessel is sealed, and then repeatedly vacuumed and filled with nitrogen. The reaction vessel after deoxygenation is subjected to polymerization reaction at a set temperature (from room temperature in the range of 20 to 100° C.). To the set time (depending on the polymer molecular weight, monomer and temperature to be controlled, the time can range from 0.5 to 24 hours), take it out and add tetrahydrofuran to dilute, and then pour it into excess methanol with 1:1 hydrochloric ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com