Agglutinant competitive process liver cancer a-fetoglobulin heteroplasmon AFP-L3 quantitative test reagent kit
A technology for AFP-L3 and alpha-fetoprotein, which is applied in the field of kits for detecting the content of alpha-fetoprotein heterogenous AFP-L3, can solve the problems of difficulty in distinguishing benign and malignant lesions, high technical requirements, complicated operation and the like, and achieves the method Simple application, high sensitivity, overcoming the effect of cumbersome operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Production of AFP-L3 ELISA Quantitative Detection Kit
[0053] A kind of AFP-L3 ELISA quantitative detection kit (96 people), its composition comprises:
[0054] One bottle of AFP-L3 negative and one positive control;
[0055] AFP monoclonal antibody coated plate (96 wells) 1 piece;
[0056] Horseradish peroxidase (HRP)-labeled AFP-L3 monoclonal antibody 1 bottle, 6ml / bottle;
[0057] Lentil agglutinin (LCA) solution 1 bottle
[0058] 1 bottle of blank buffer
[0059] 1 bottle of AFP standard
[0060] 1 bottle each of substrate solution A and chromogenic solution B, each 5ml / bottle;
[0061] 1 bottle of reaction termination solution, 5ml / bottle;
[0062] Wash buffer (20X concentrated) 1 bottle, 30ml / bottle.
[0063] The specific operation is as follows:
[0064] 1. Prepare AFP-negative and positive controls:
[0065] 1) Serum collection: Obtain the serum of healthy normal people and ascites of liver cancer patients from the hospital or blood bank, an...
Embodiment 2
[0109] Example 2: Use of AFP-L3 Quantitative ELISA Kit
[0110] 1. Washing buffer preparation: dilute the concentrated washing buffer provided in the kit with distilled water 20 times.
[0111] 2. Antigen-antibody reaction: Add 50 μl of positive or negative quality control serum, or serum samples to be tested, to the microwells of the antibody-coated plate provided in the kit, and incubate in a water bath at 37°C for 30 minutes, wash the plate 5 times, Add lectin solution and control solution to each double well, incubate in 37°C water bath for 30 minutes, shake off the liquid, add HRP-monoclonal antibody solution to each well, 50ul per well, incubate in 37°C water bath for 30 minutes. Repeat the plate washing operation 5 times, 3 minutes each time.
[0112] 3. Color reaction: Add 50 μl each of substrate solution A and TMB chromogenic solution B to each well in turn, incubate in a 37°C water bath for 10 minutes, and then add 50ul of reaction termination solution to each well ...
Embodiment 3
[0121] Example 3: Detection of positive samples of liver cancer patients by the AFP-L3 ELISA quantitative detection kit of the present invention
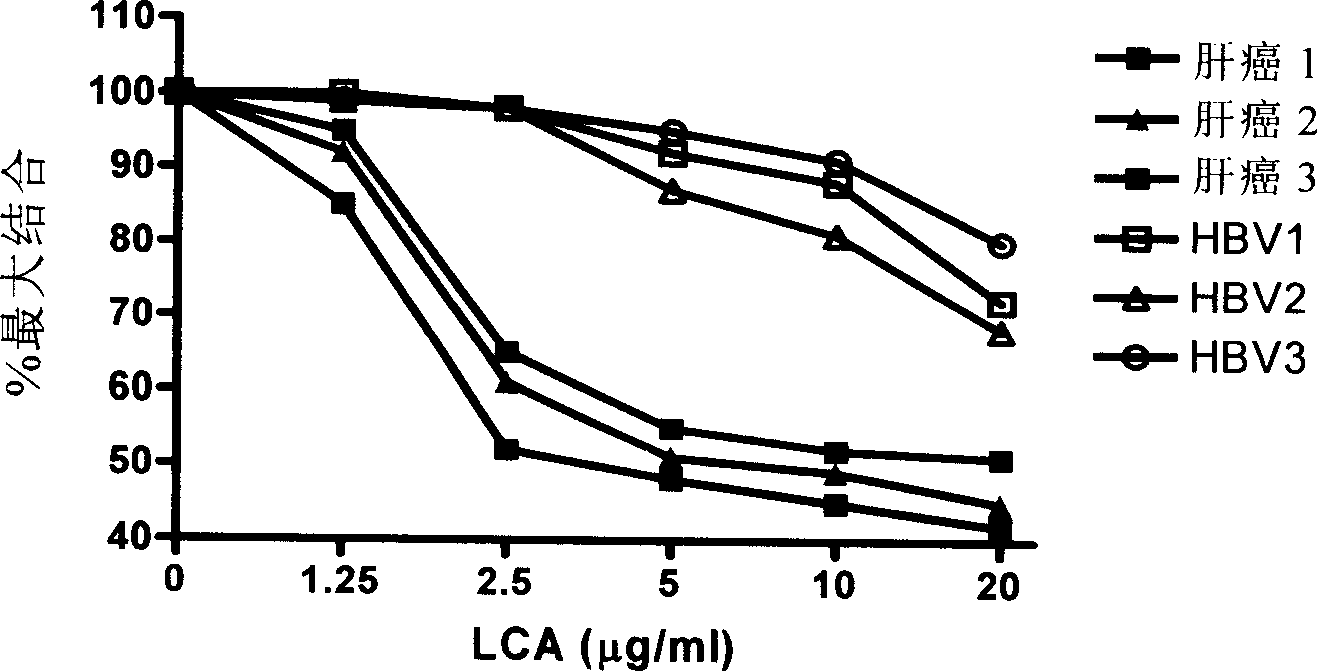
[0122] In order to judge the coincidence rate between the AFP-L3 ELISA quantitative detection kit of the present invention and the clinical liver cancer detection results, the present invention is used for detection. Clinical research data of Ditan Hospital: 100 known positive specimens of primary liver cancer, 50 healthy serum samples from physical examination, and 100 liver cirrhosis serum samples were tested with AFP-L3 enzyme-linked immunosorbent assay kit. The results are shown in Table 1.
[0123] serum source
[0124] The results of this experiment show that the coincidence rate of the present invention for primary liver cancer is as high as 82%, and the specificity for liver cirrhosis is as high as 95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com