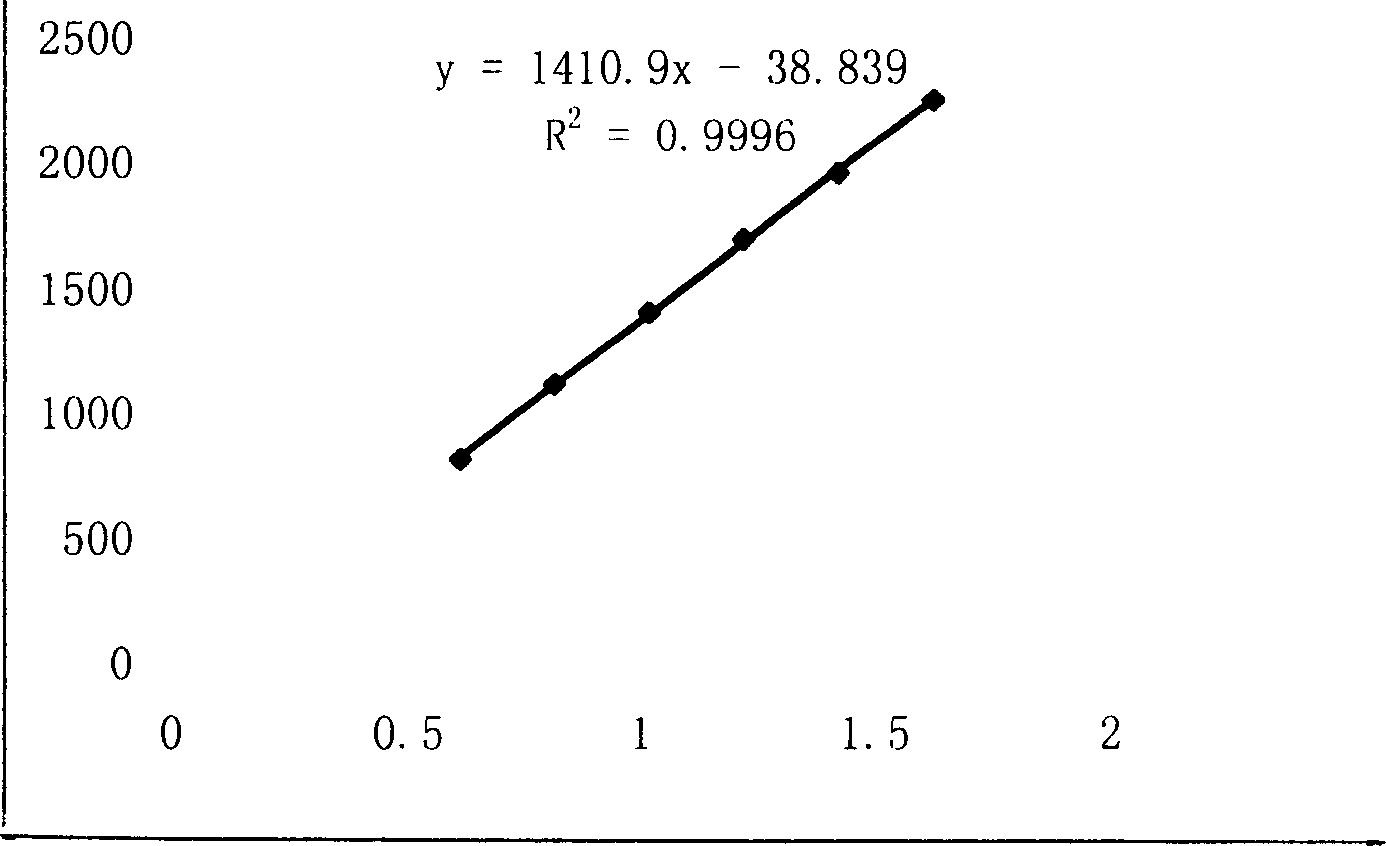Preparation method of 'Xue Fu Zhu Yu' capsule and quality standard thereof
A technology of removing blood stasis from blood and quality standards, applied in the directions of capsule delivery, medical preparations containing active ingredients, measuring devices, etc. problem, to achieve the effect of good reproducibility, rapid and accurate detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0239] Example 1 Improved Xuefu Zhuyu Capsule Quality Standard
[0240] 1. Name: Xuefu Zhuyu Capsules
[0241] 2. Prescription:
[0242] Peach kernel (stir-fried) Angelica aurantium husk (stir-fried with bran) Chuanxiong
[0243] Bupleurum safflower Achyranthes bidentata Radix Paeoniae Rubra
[0244] Rehmannia glutinosa Licorice
[0245] 【Properties】This product is a capsule, and the content is a brown powder; the gas is pungent, and the taste is slightly bitter.
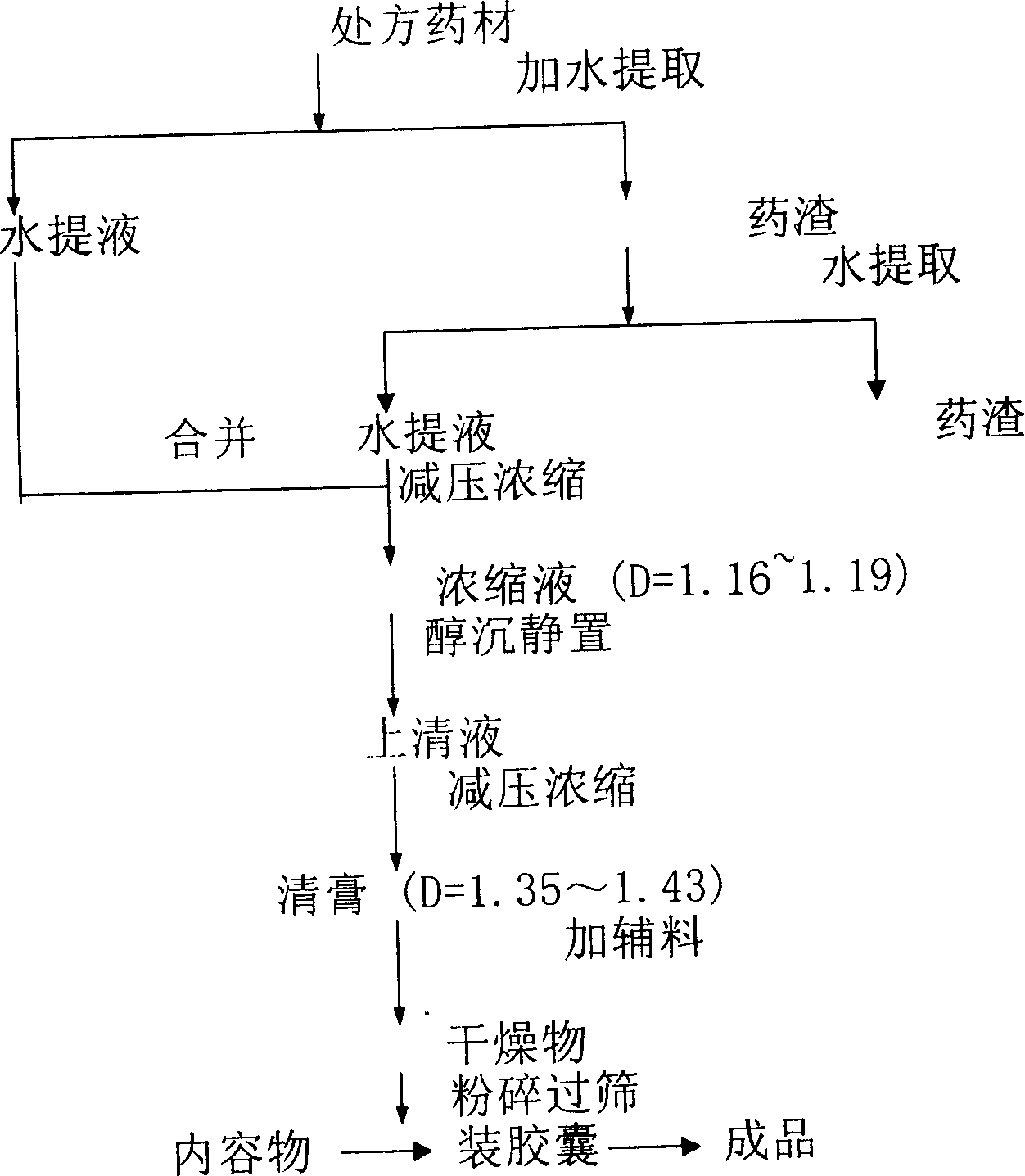
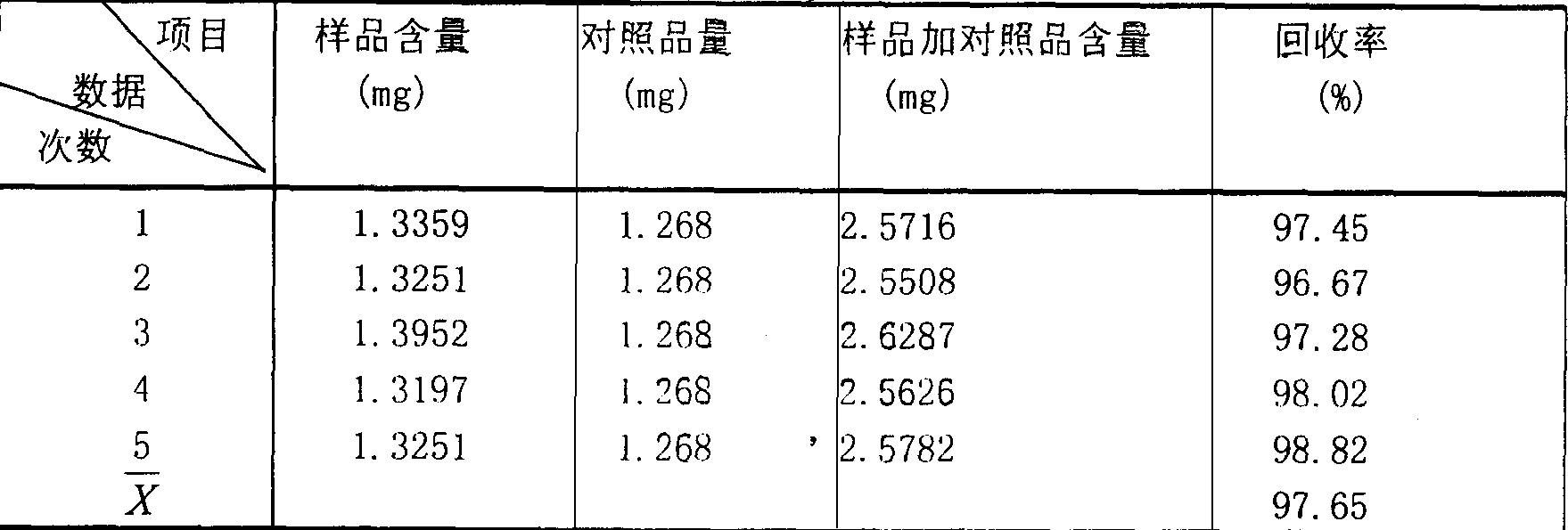
[0246] [Preparation method] Extract 11 medicinal herbs such as angelica with water for 1-3 times, use 6-10 times the amount of water, filter for 0.5-1.5 hours each time, collect the filtrate, and concentrate under reduced pressure to a relative density of 1.07-1.35 ( 60-70°C), leave it at room temperature, add ethanol to make the alcohol content 60-80%, stir well, let it stand (5-25h) to precipitate, take the supernatant and concentrate it under reduced pressure to a relative density of 1.30-1.50 (60-70°C) add ...
Embodiment 2
[0281] Take 1.35kg of eleven herbs such as angelica, extract with water twice, each time with 8 times the water consumption, each extraction time is 1h, filter, collect the filtrate, concentrate under reduced pressure to a relative density of 1.16 (60°C), and place it in the cold At room temperature, add ethanol to make the alcohol content 70%, stir well, let it stand for precipitation, take the supernatant and concentrate it under reduced pressure to a relative density of 1.402 (60°C), add 61.5g of starch, and dry it in a drying oven for 9h ( T=70-75° C.) to obtain 378 g of dry matter, add 22.68 g of silicon dioxide, pulverize through a 40-mesh sieve, pack into capsules, and make 1000 grains. (Each gram of capsule is equivalent to 3.375g of crude drug)
Embodiment 3
[0283] Take 1.35 kg of eleven herbs such as angelica, extract with water 3 times, each time with 6 times the water consumption, each extraction time is 0.5h, filter, collect the filtrate, concentrate under reduced pressure to a relative density of 1.08 (60°C), and wait for cooling Leave it at room temperature, add ethanol to make the alcohol content 60%, stir well, let it stand for precipitation, take the supernatant and concentrate it under reduced pressure to a relative density of 1.43 (60°C), add 64.8g of starch, and dry in a drying oven for 9 hours (T=65-70° C.), to obtain 375 g of dry matter, add 25.53 g of silicon dioxide, pulverize through a 24-mesh sieve, pack into capsules, and make 1000 grains. (Each gram of capsule is equivalent to 3.215g of crude drug)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com