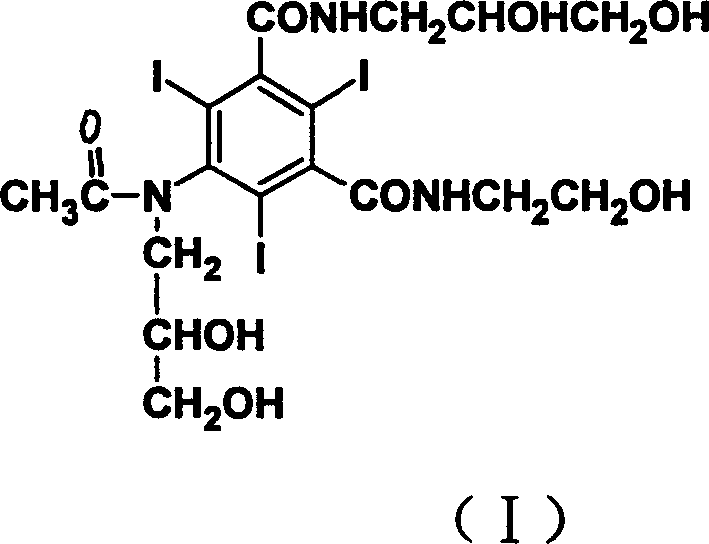
Iodine Ioxilan preparation method
A technology of triiodo-m-formamide and dihydroxypropyl, which is applied in the field of preparation of ioxilan, can solve the problems of low conversion rate, complex post-treatment, similar chemical structure of impurity oxyalkyl compounds, etc., and achieve the goal of reducing damage Possibility, shortening of reaction steps, effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
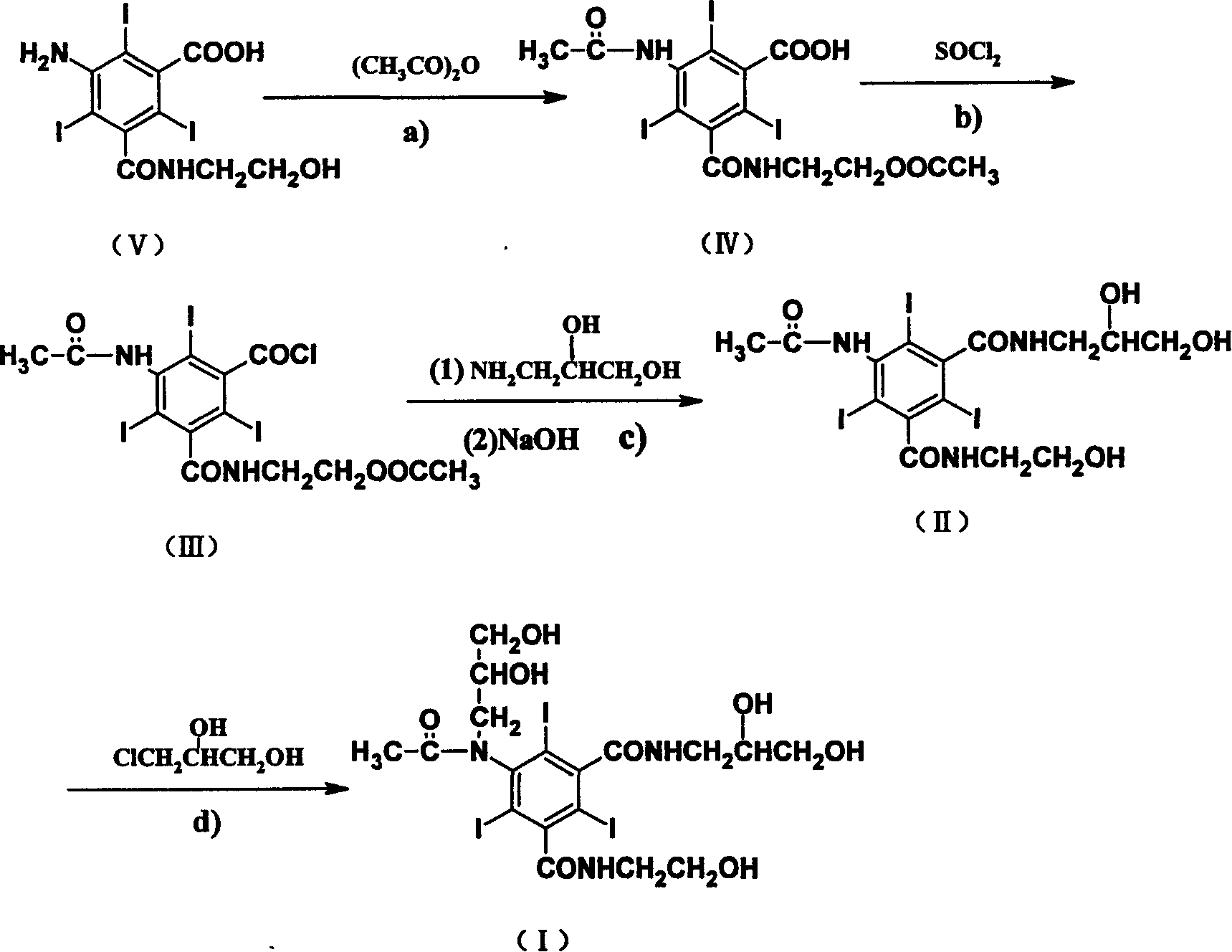
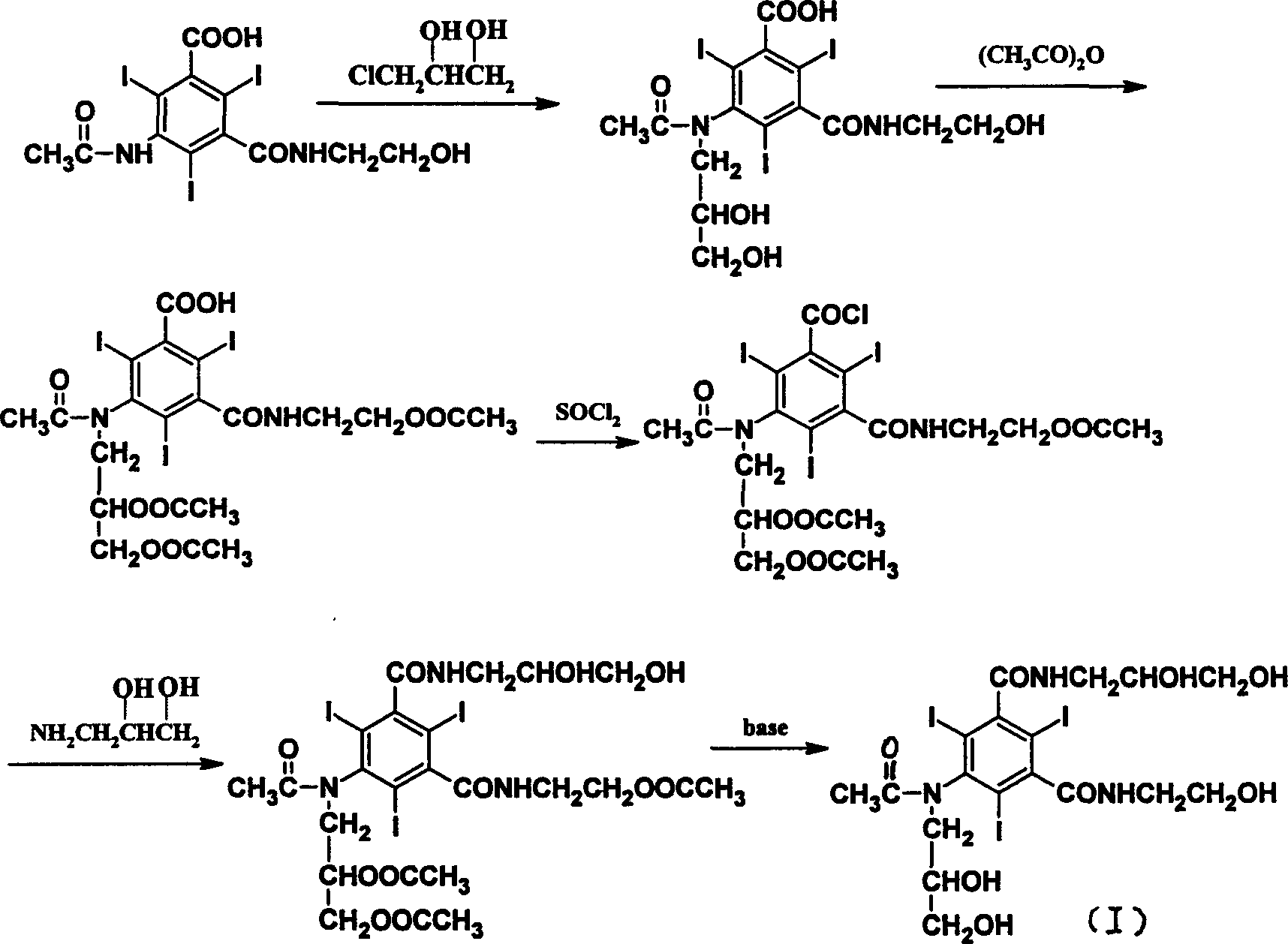
[0024] Synthesis of 5-acetamido-N-(2-acetoxyethyl)-2,4,6-triiodo-m-carboxamide benzoic acid:
[0025]In a three-necked flask equipped with a stirrer and a reflux condenser, add 80g (0.133mol) of 5-amino-N-(2-hydroxyethyl)-2,4,6-triiodo-m-formamide benzoic acid and 300mL (3.18mol) acetic anhydride, after stirring evenly, add 0.36mL (0.004mol) perchloric acid dropwise, then raise the temperature to the acylation reaction temperature of 50°C, stir for 25 hours to end the reaction, and use 0.33g (0.004mol) acetic acid Sodium to neutralize perchloric acid. After acetic anhydride and acetic acid are evaporated under reduced pressure, the residue can be directly used for the next step (Example 3 or 4) without purification.
Embodiment 2
[0027] The acylation reaction temperature was 90° C., and the reaction time was 10 hours. The rest of the operations were the same as in Example 1.
Embodiment 3
[0029] Synthesis of 5-acetamido-N-(2-acetoxyethyl)-2,4,6-triiodo-m-formamide benzoyl chloride:
[0030] Dissolve the 5-acetamido-N-(2-acetoxyethyl)-2,4,6-triiodo-m-formamide benzoic acid obtained in the previous step (Example 1) in 250 mL (2.53 mol) at room temperature In ethyl acetate, after stirring evenly, add 29 mL (0.4 mol) of thionyl chloride, then raise the temperature to the acid chloride reaction temperature of 50°C, and stir for 6 hours to complete the reaction. After ethyl acetate and thionyl chloride were evaporated under reduced pressure, 100 mL of ethyl acetate was added to the residue, and the solvent was evaporated again. The obtained residue could be directly used in the next step (Example 5 or 6) without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com