Anticancer diterpene analog compound intravenous injection formulation, and its preparation method and uses
A technology of compound and diterpenoids, which is applied in the field of anticancer diterpenoids intravenous injection and its preparation, can solve the problems of poor solubility and low bioavailability of anticancer diterpenoids, and achieve enhanced anticancer efficacy , reduce the dosage, improve the effect of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, preparation anticancer diterpene compound intravenous injection
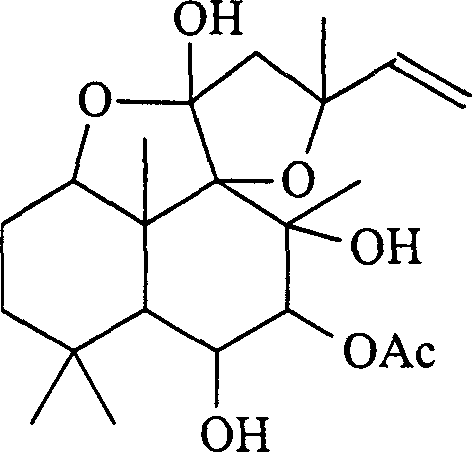
[0044] The diterpenoid compound shown in the formula I of anticancer effective amount is dissolved in the pharmaceutically acceptable compound solvent according to the following steps:
[0045] Described composite solvent comprises by weight percent:
[0046] Molecular inclusion carrier: Captisol (SBE-β-CD) 40wt%
[0047] Osmotic pressure regulator: NaCl 0.9wt%
[0048] Polar solvent: absolute ethanol 5wt%
[0049] Semi-polar solvent: propylene glycol 5wt%
[0050] Non-polar solvent: ethyl acetate 5wt%
[0051] Antioxidant: sodium sulfite 0.5wt%
[0052] Acidity (pH) adjuster: 8wt% NaHCO 3 Appropriate amount, adjust the pH of the mixture to 7.2
[0053] The balance is deionized triple distilled water
[0054] 1) first dissolving the diterpenoids and antioxidants shown in the formula I of anticancer effective amount in polar solvents, semipolar and nonpolar solvents to obtain a transp...
Embodiment 2
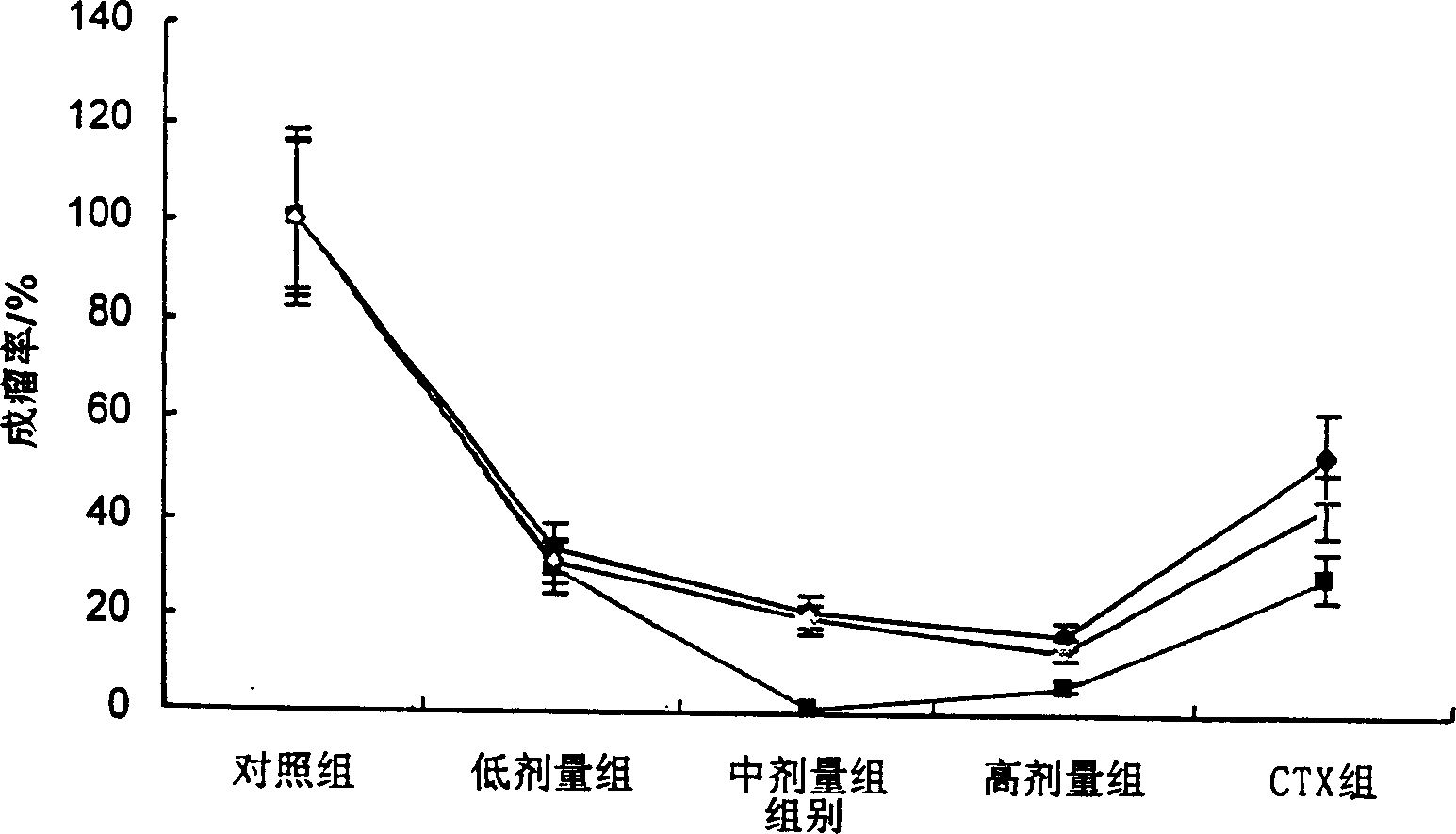
[0058] Example 2. In vivo anti-tumor experiment of diterpenoids on human nasopharyngeal carcinoma (CNE) xenograft tumor in nude mice
[0059]Test drug: the diterpenoid intravenous injection prepared in Example 1, wherein the diterpenoid was prepared and purified by the State Key Laboratory of Biofilm and Membrane Engineering, Institute of Zoology, Chinese Academy of Sciences, with a purity of more than 99%, and it is a white crystal , stable physical and chemical properties, prepared with nano-inclusion preparations.
[0060] Positive control: Cyclophosphamide (CTX), a product of Jiangsu Hengrui Pharmaceutical Co., Ltd., approved by the National Pharmaceutical Standard H32020857, prepared with normal saline.
[0061] Negative control: Molecular carrier inclusion agent (no drug, only the compound solvent of the aforementioned test drug), provided by the Institute of Zoology, Chinese Academy of Sciences
[0062] Cell model: human nasopharyngeal carcinoma cell (CNE), provided by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com