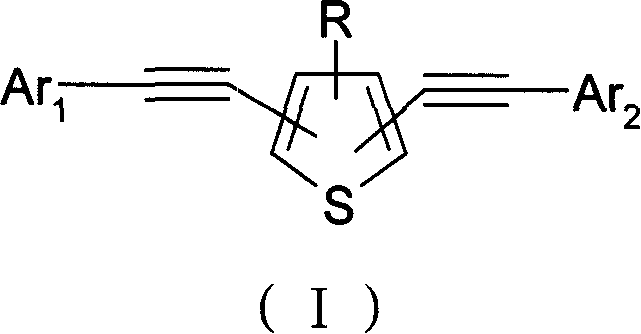
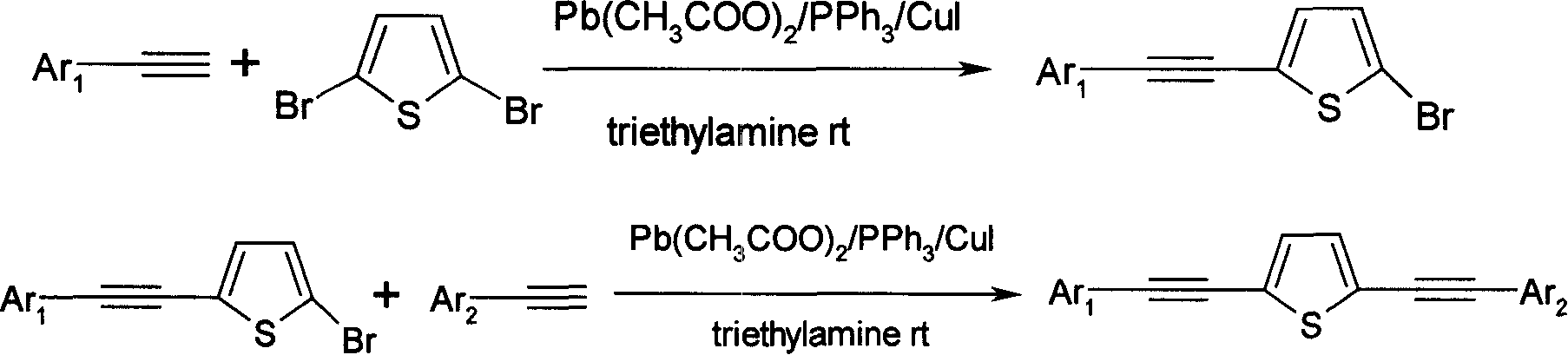
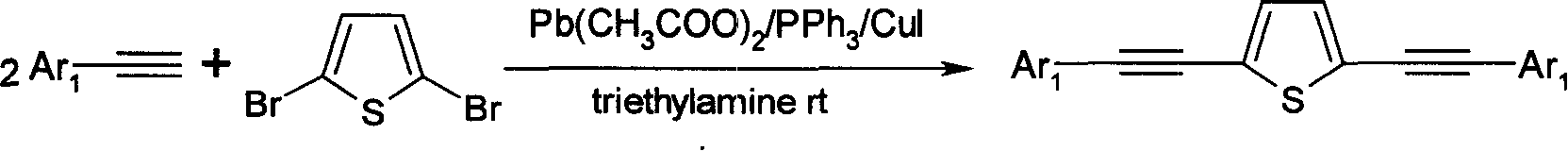Alkynyl thiofuran compound and its prepn and application
An alkynyl thiophene and compound technology, applied in the field of alkynyl thiophene compounds, can solve the problems of failure to popularize and apply, limited application value, low cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Preparation of 2,5-bis(thiophen-2-ylethynyl)thiophene
[0052] Weigh 10.8g (0.1mol) of 2-ethynylthiophene and 12.1g (0.05mol) of 2,5-dibromothiophene, add 100ml of anhydrous diethylamine, stir under the protection of nitrogen, add 0.0325g (0.0001mol) of palladium acetate ), cuprous iodide 0.038g (0.0002mol) and triphenylphosphine 0.0786g (0.0003mol). The temperature was raised and refluxed for 4h, and a large amount of salt was produced. Cool to room temperature, filter, and wash the remaining solid with benzene; combine the filtrate, distill under reduced pressure to remove the solvent, and recrystallize with ethanol to obtain 13.26 g of 2,5-bis(thiophen-2-ylethynyl) as pale yellow flake crystals Thiophene has a melting point of 113.8-114.3°C. 1 HNMR (500MHz): δ7.3410 (d, J=5.05Hz, 2H, thiophene-H), 7.3135 (d, J=3.45Hz, 2H, thiophene-H), 7.305 (d, J=8.8Hz, 2H, thiophene-H), 7.1578(s, 2H, thiophene-H). 13 C NMR: δ85.84, 87.51, 122.65, 124.65, 127.24, 128.04, 131....
Embodiment 2
[0053] Example 2: Preparation of 2,5-diphenylethynylthiophene
[0054] -40℃, 16gNaNH 2 Add to 200mL liquid ammonia, add 20.4kg phenylacetylene, and pass N 2 Protect, add 24.2g dibromothiophene in ether solution, heat to -30℃ with stirring, stop stirring after 1h, let stand overnight, add NH 4 Cl, heated to recover ammonia gas, filtered, washed with ether solution, removed ether, and dried to obtain a yellow solid, recrystallized from ethanol, 22.5 g of pale yellow flake solid, with a yield of 80%. mp 75.8-76.7°C. 1 H NMR (500MHz): 87.1539 (s, 2H, thiophene-H), 7.32-7.53 (m, 10H, Ph-H). 13 C NMR: δ 82.34, 94.12, 122.73, 124.78, 128.44, 128.69, 131.55, 131.83. EIMS m / z (%): 284 (M + , 100), 158(3), 142.05(21).
[0055] To synthesize asymmetric 2,5-diynylthiophene compounds, the reaction formula is as follows:
[0056]
Embodiment 3
[0057] Example 3: Preparation of 2-pyridine-3-ethynyl-5-p-methoxyphenylethynylthiophene:
[0058] Weigh 10g (0.097mol) of meta-ethynylpyridine and 24.2g (0.1mol) of 2,5-dibromothiophene, add 100ml of anhydrous triethylamine, stir under the protection of nitrogen, add 0.065g (0.0002mol) of palladium acetate, Cuprous iodide 0.076g (0.0004mol) and triphenylphosphine 0.1572g (0.0006mol). The temperature was raised and refluxed for 4h, and a large amount of salt was produced. Cool to room temperature, filter, and wash the remaining solid with benzene; combine the filtrate and distill off the solvent under reduced pressure to obtain 21 g of 1-pyridin-3-yl-2-(5-bromothiophen-2-yl)acetylene as a solid, with a melting point of 128°C.
[0059] Weigh 6.6g (0.05mol) of p-methoxyphenylacetylene and 13.2g (0.05mol) of 1-pyridin-3-yl-2-(5-bromothiophen-2-yl)acetylene, add 100ml of anhydrous triethylamine , Stirring under the protection of nitrogen, add 0.0325g (0.0001mol) of palladium acetate, 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com