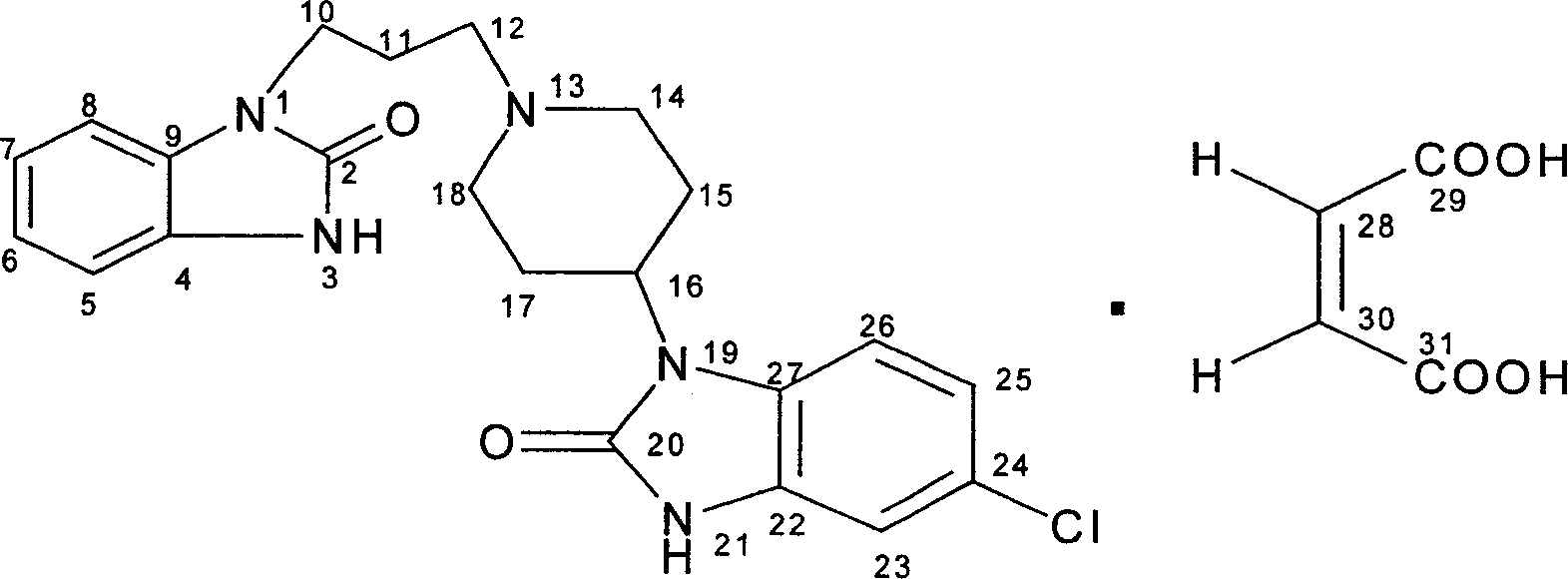
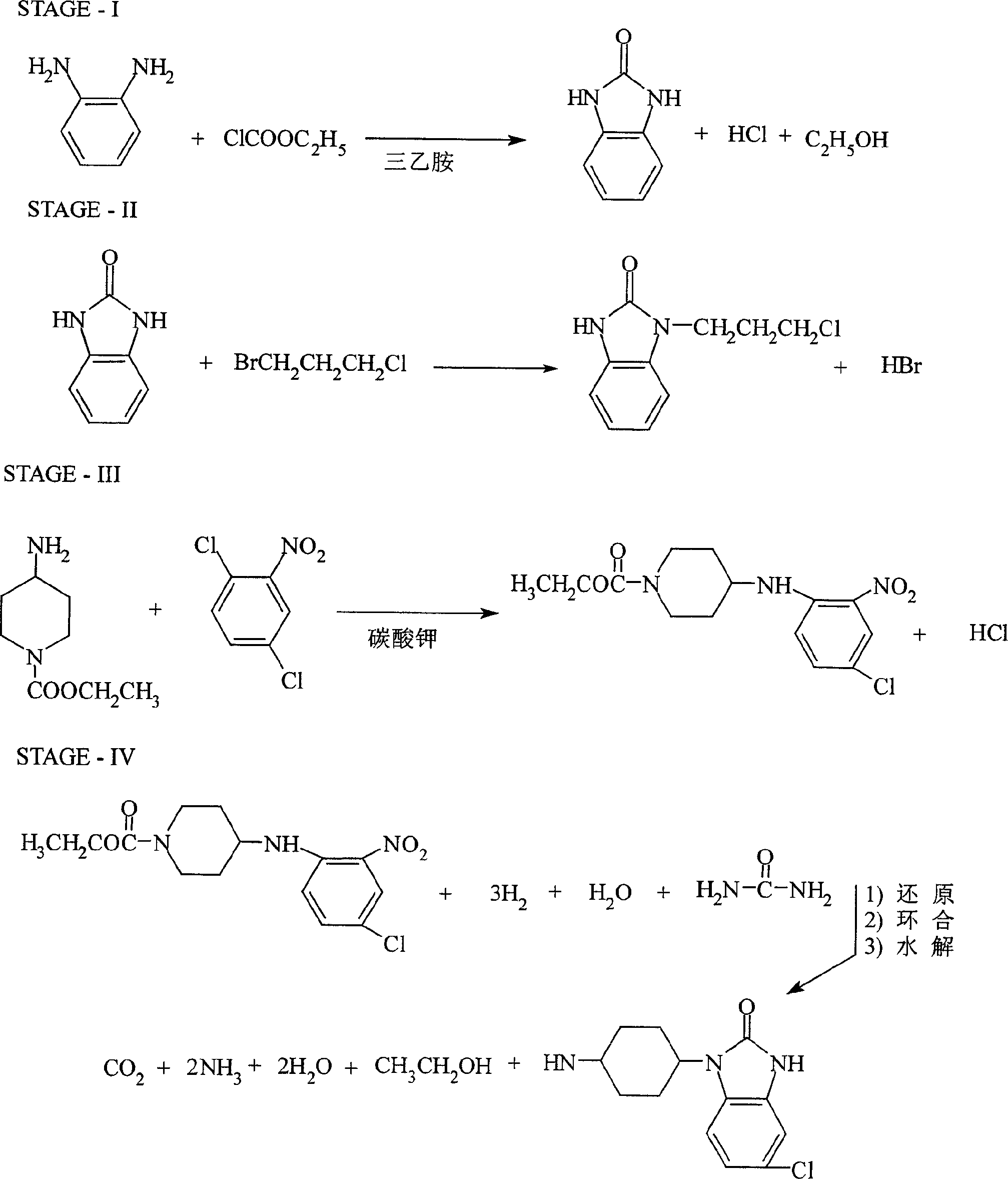
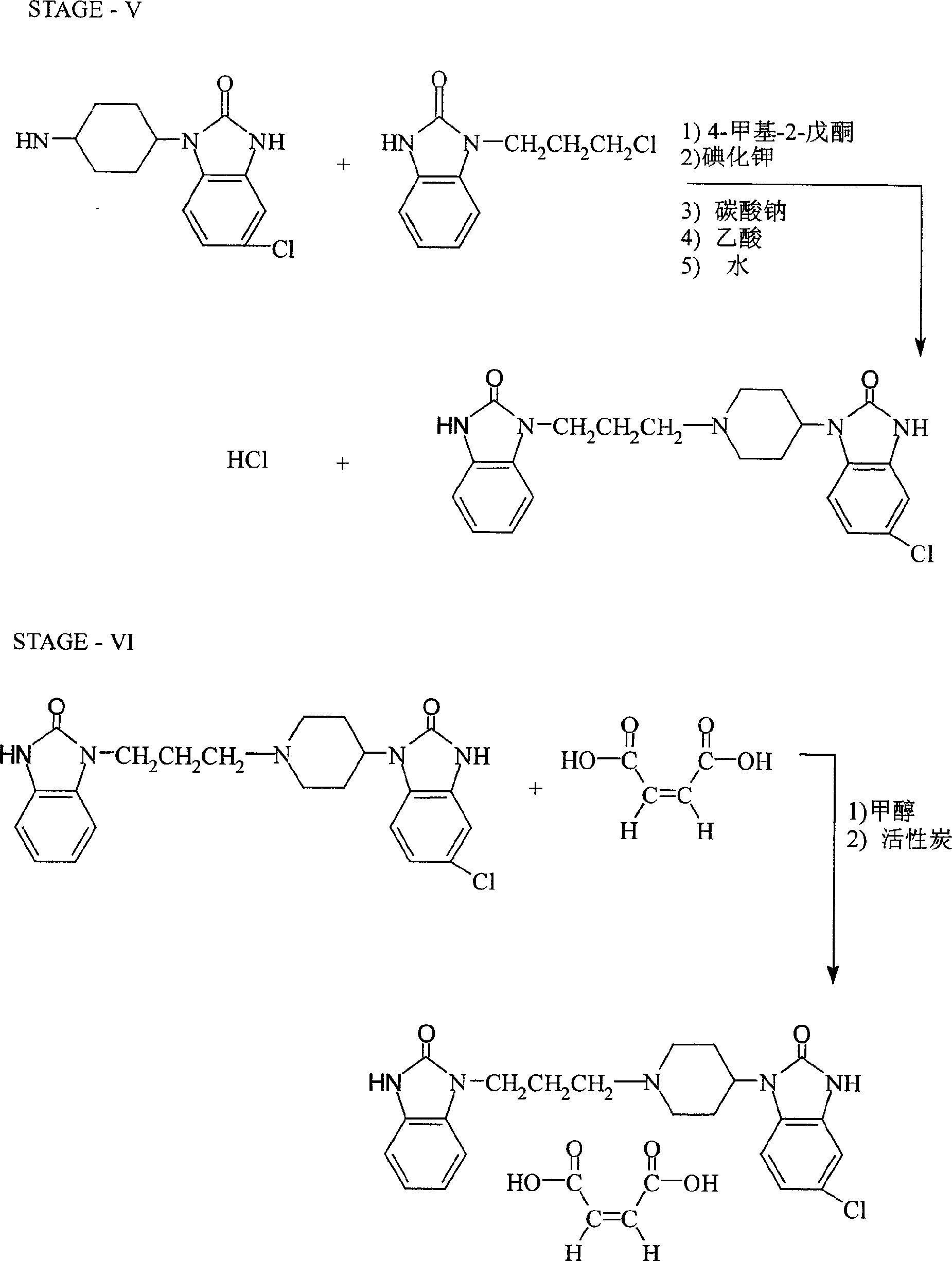
Synthesis of domperidone maleate
A technology for domperidone maleate and its synthesis method, which is applied in the directions of pharmaceutical formulations, organic active ingredients, and medical preparations containing active ingredients, etc., and can solve the problems of complex production process, high cost, and low yield of domperidone maleate, Achieve the effect of being convenient for industrialized production, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0045] The following examples are intended to illustrate certain preferred embodiments of the present invention and are not intended to limit the scope of the present invention.
[0046] 1, Synthesis of 2(3H) benzimidazolone (I)
[0047] Add 448g of o-diaminobenzene and 1000ml of toluene into a 2.5L reaction flask, raise the temperature to 60-70°C, within 6 hours, slowly add 496g of ethyl chloroformate and 502g of triethylamine dropwise at the same time, and control the pH value at Between 8 and 11, keep the reaction for 6 hours under the condition of 60-70°C after dropping. After cooling to 5~-10°C, a solid was precipitated, filtered, washed with water, and dried to obtain 513g of product with a melting point of >300°C and a yield of 92.1%.
[0048] 2. Synthesis of 1-(3-chloropropyl)-1,3-dihydro-2H-benzimidazol-2-one (II)
[0049] Add 446g of I, 577g of 1-bromo-3-chloropropane and 1340ml of 10% sodium hydroxide solution in the reaction flask, stir and dissolve at room tempe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com