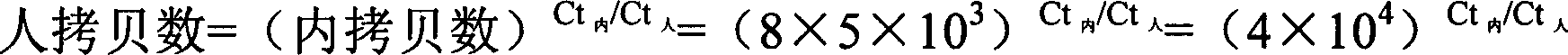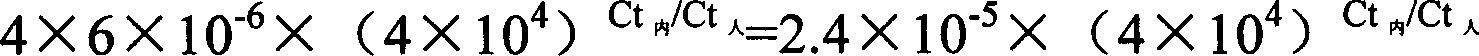Huaman plasma DNA quantitative analyser
A technique for quantitative analysis of human plasma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] 1. Preparation of Exogenous DNA
[0031] Insert the oligonucleotide with A at the 3' end into the vector pMD18T to prepare competent bacteria, and transfer the whole amount of pMD18-T containing the target fragment into 200 μl of competent bacteria; after blue-white screening and colony PCR identification, it is positive Cloning and extraction of plasmid DNA containing the target fragment.
[0032] Restriction endonuclease Hind II digests the plasmid internal reference to linearity: After digestion, dilute the linear cloned plasmid DNA internal reference to 1×10 3 copies / μl.
[0033] 2. Plasma Separation and Storage
[0034] with EDTA-K 2 Extract 3ml of human peripheral blood from a 5ml sterile plastic anticoagulant centrifuge tube with a cover, and centrifuge at 2000r / min for 20min within 4h at room temperature to collect plasma; centrifuge again at 3000r / min for 15min to obtain blood cell-free plasma, take 0.5ml and collect To which was added a concentration of 1×...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com