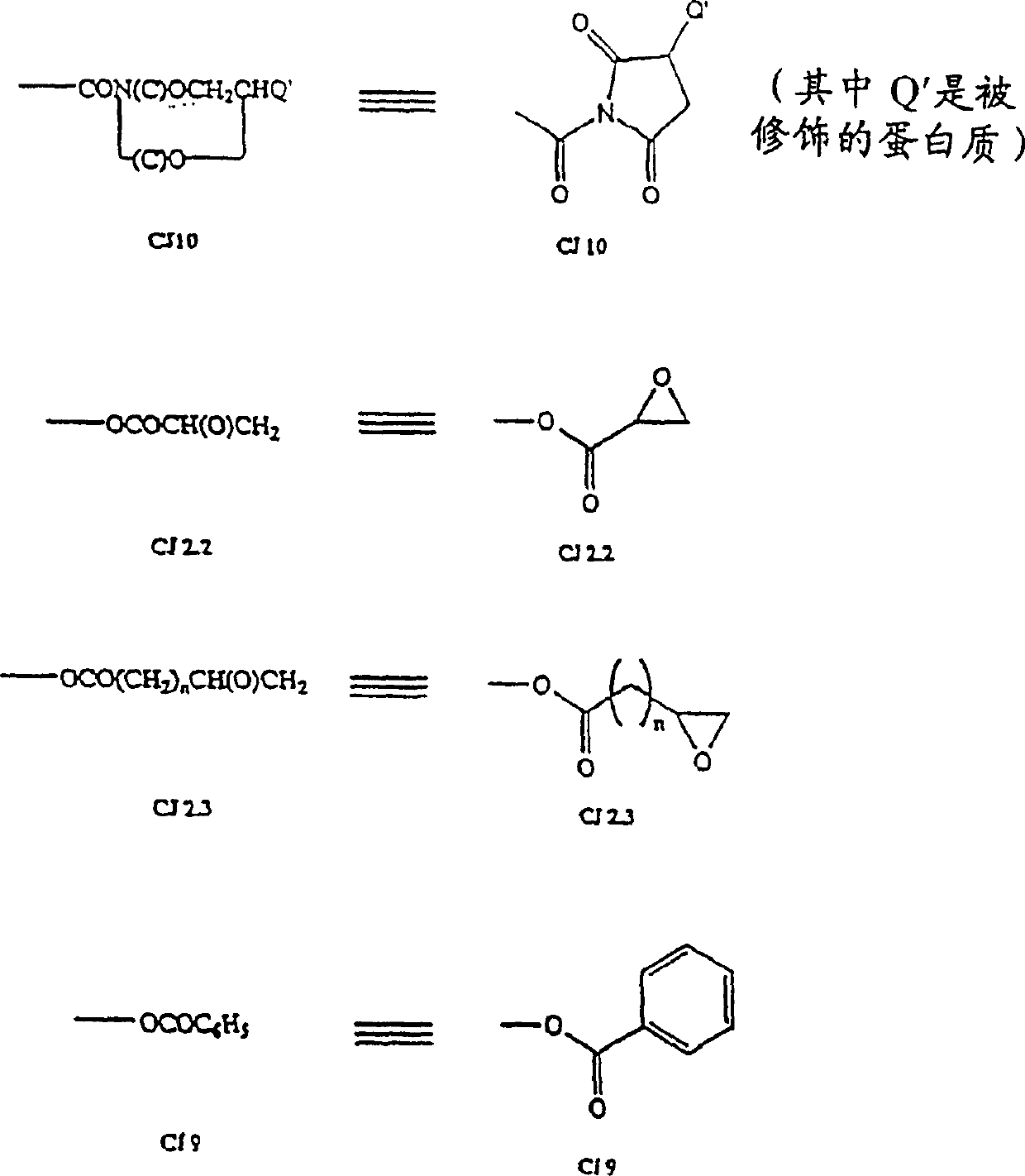
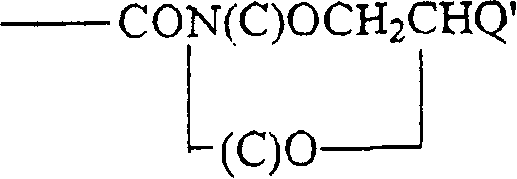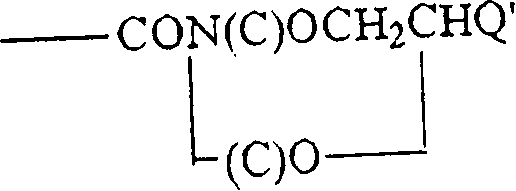Hapten-carrier conjugates for use in drug-abuse therapy and methods for preparation of same
A hapten and conjugate technology, which can be used in drug combinations, carrier-binding antigen/hapten components, antibodies, etc., to solve the problems of complex neurochemical events, insufficient to overcome addiction, and limited efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0144] Example 3, Method A).
[0145] Conjugate
Conjugation method
PS-54
PS-54
PS-55
PS-55
PS-56
PS-56
PS-57
PS-57
PS-58
BSA
HEL
BSA
HEL
BSA
HEL
BSA
HEL
BSA
19.5
3.2
33.2
1.09
27
2.2
81
8
66.8
Example 1, Method B
Example 1, Method B
Example 3, Method A
Example 3, Method A
Example 3, Method A
Example 3, Method A
Example 3, Method A
Example 3, Method A
Example 3, Method A
PS-58
HEL
7.4
Example 3, Method A
[0146] This is a non-limiting list of conjugates. Other conjugates are made with more than one hapten linked to a carrier containing T cell epitopes. Preferably, 1-100 haptens are linked to a carrier containing T cell epitopes. More preferably, 1-70 haptens are linked to a carrier containing T cell epitopes.
[0147] The examples disclose the synthesis methods of the compounds PS-54, P...
Embodiment 1
[0190] Preparation of nicotine conjugate
[0191] Method A To a solution of nornicotine (50 mmol) in dichloromethane was added triethylamine (75 mmol) followed by succinic anhydride (100 mmol). The solution was heated at reflux for 18 hours. The reaction mixture was washed with 10% aqueous hydrochloric acid, saturated sodium bicarbonate solution, brine and water in this order. Dry (MgSO 4 After removing the solvent under reduced pressure, the residue is purified by silica gel flash chromatography to obtain the expected product.
[0192] Method B The succinylated nornicotine was then used to synthesize the nicotine conjugate PS-54 (Figure 7). To the succinylated nornicotine (5 μm) DMF solution (0.1 ml), diisopropylethylamine (10 μm) and HATU (5.5 μm) were sequentially added. After 10 minutes, the light yellow solution was added dropwise to a solution (0.9 ml) of HEL or BSA (500 μg) in a 0.1 M sodium borate buffer at pH 8.8, and the mixture was stirred at room temperature for 18 hou...
Embodiment 2
[0210] Method A (S)-N’-butyric acid adduct of nicotine
[0211] Under argon, at ice water temperature, ethyl 4-bromobutyrate (0.0341 mol) was added dropwise to (S)-nicotine (0.031 mol) in anhydrous methanol solution (50 ml) within 10 minutes. The resulting orange solution was returned to room temperature and stirred for 18 hours. The solvent was removed under reduced pressure, and the remaining brown residue was precipitated with hexane to obtain an analytically pure sample of the expected ester.
[0212] The ester (36mg) was dissolved in methanol (3ml) and 1M sodium hydroxide solution (5ml) and stirred at room temperature for 18 hours. The solvent was removed under reduced pressure, and the residue was dissolved in 10% hydrochloric acid and extracted with ethyl acetate. Dry (MgSO 4 After ), the solvent was removed under reduced pressure to obtain the expected compound.
[0213] Method B (S)-N'-valeric acid adduct of nicotine
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com