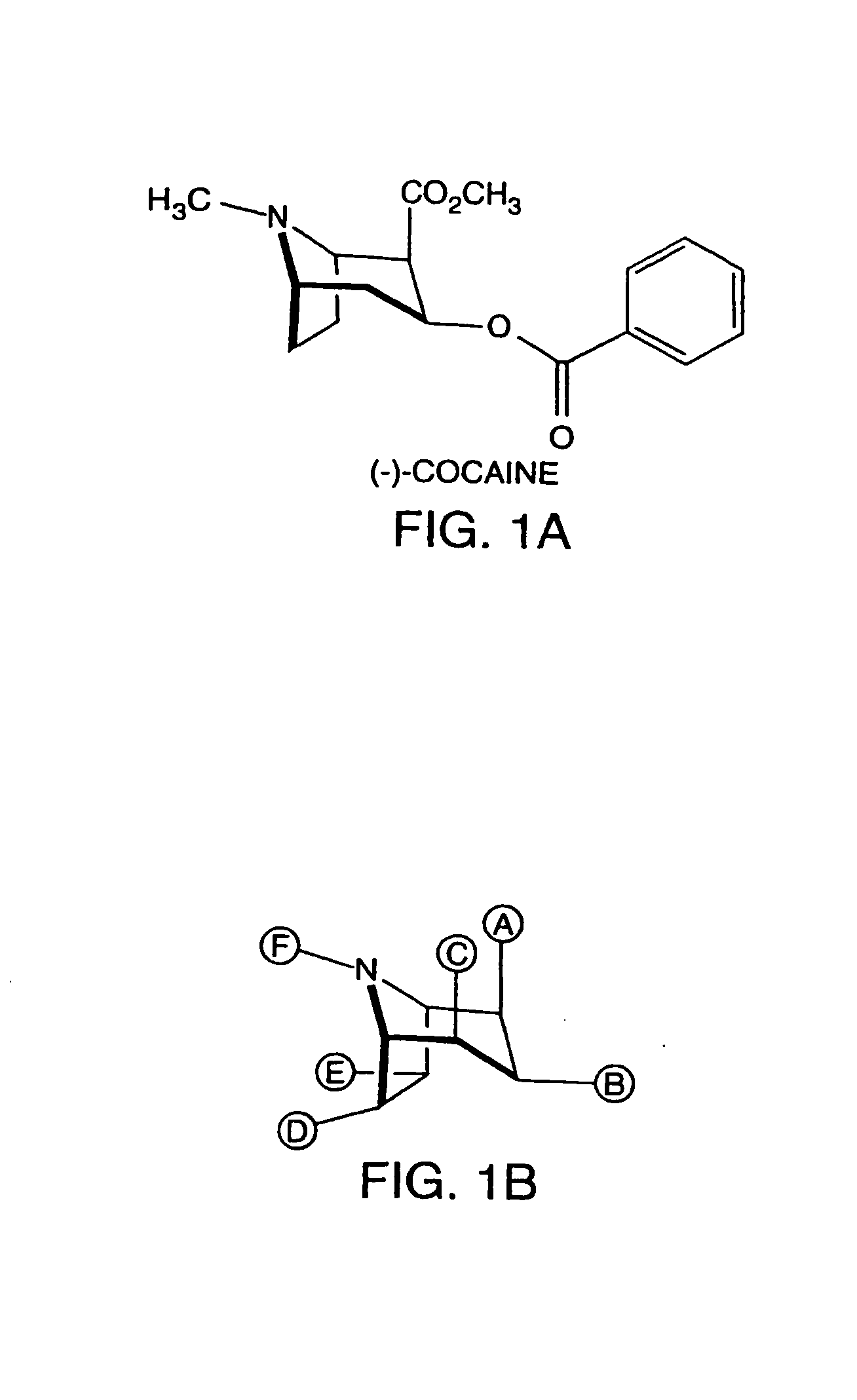
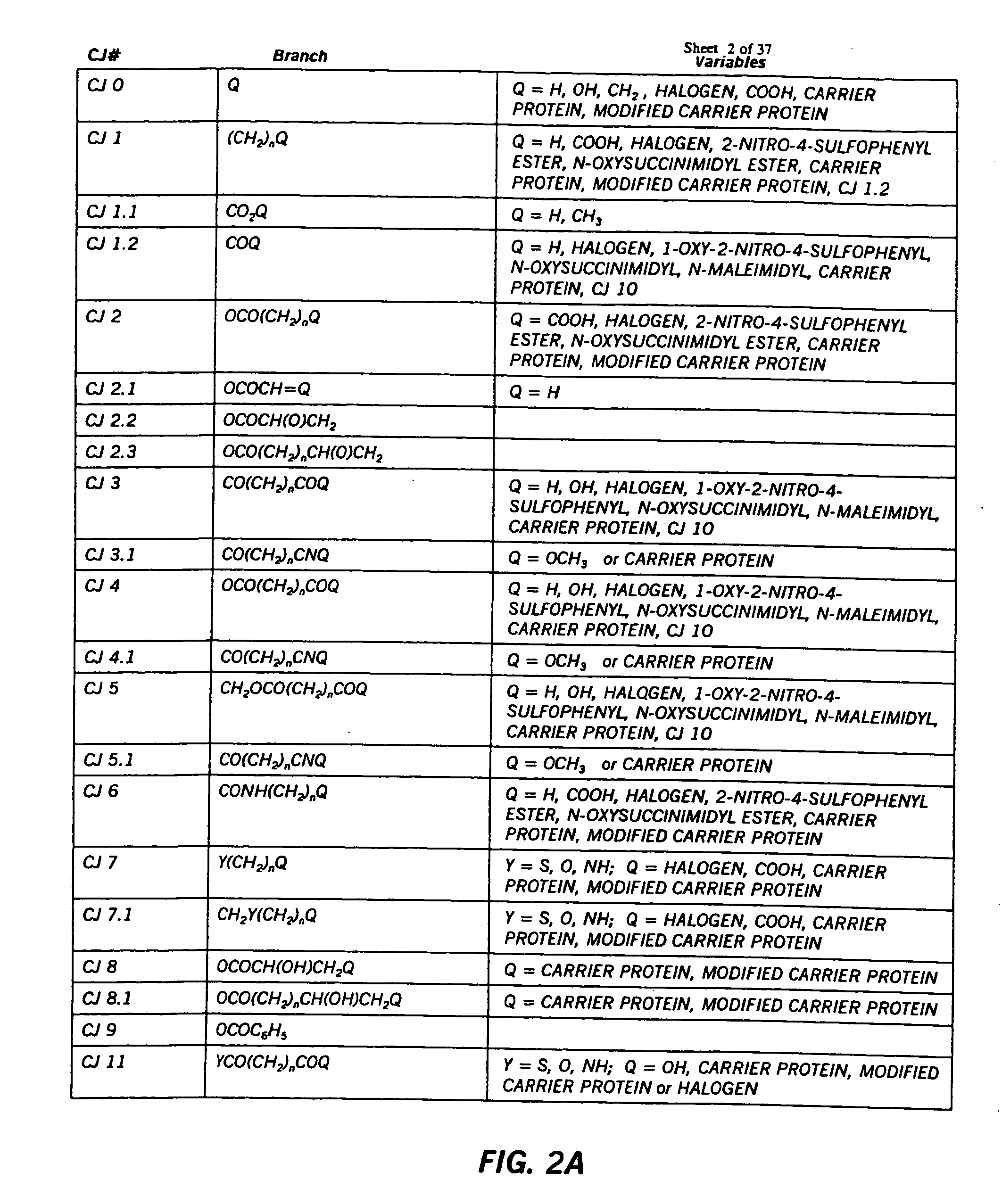
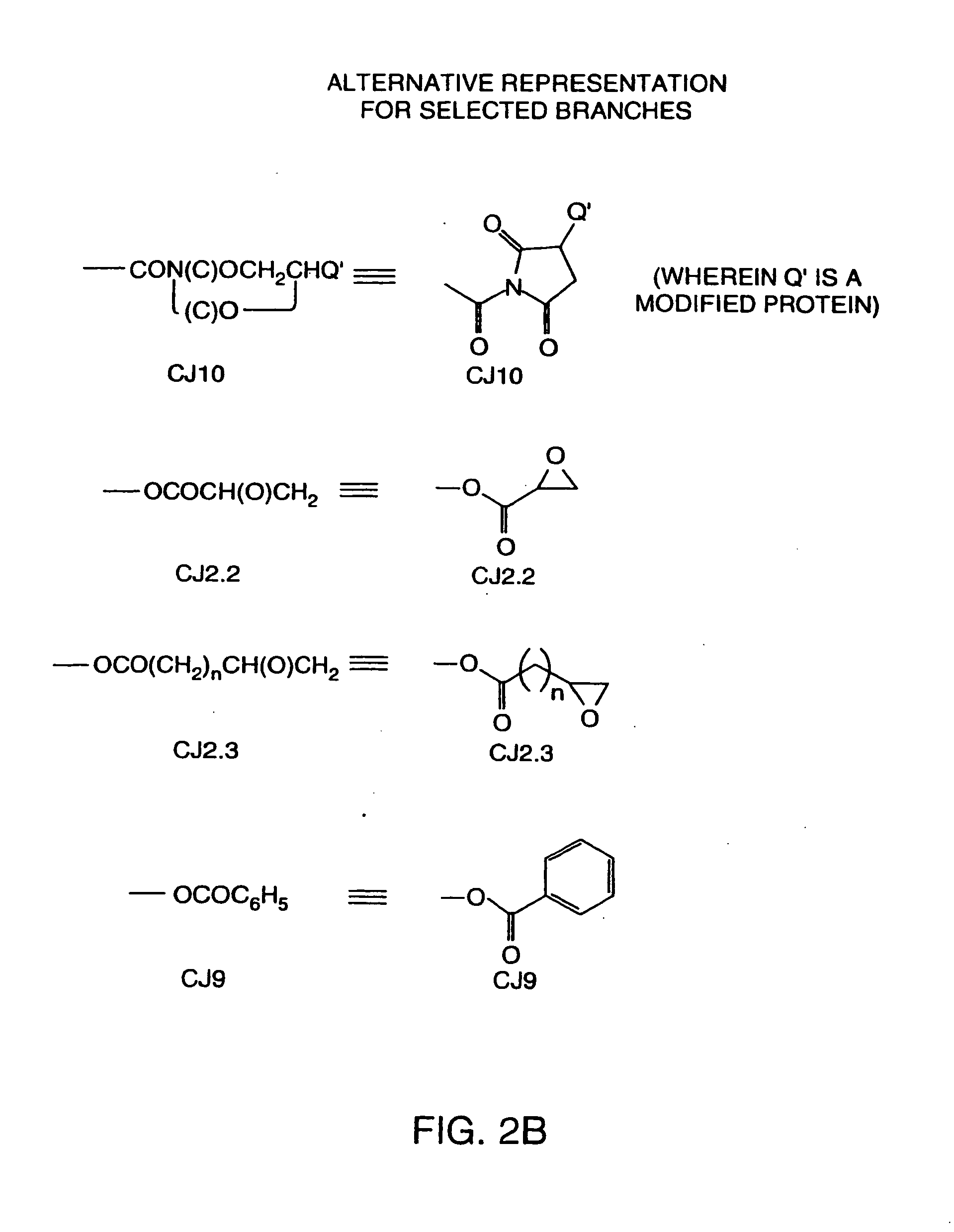
Hapten-carrier conjugates for use in drug-abuse therapy and methods for preparation of same
a technology of haptencarrier and conjugate, which is applied in the field of drug abuse treatment, can solve the problems of cocaine abuse, drug abuse is an international problem, and the individual productivity loss is an extremely high risk, and achieves the effects of stimulating the production of anti-hapten antibodies, reducing expected pharmacological effects, and high anti-drug antibody titers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of PS-2
[0192] A solution of ecgonine methyl ester hydrocholoride (50 mg, 0.21 mmol), diisopropylethylamine (80 μl, 0.46 mmol) in DMF (3 ml) was treated with bromoacetyl bromide (22 μl, 0.25 mmol) and heated at 40° C. overnight. The solvents were removed under reduced pressure and the residue purified by silica gel flash chromatography (9:1 chloroform:methanol as the eluent), furnishing the bromo compound (67 mg, 96%) as a pale yellow powder (3β-(Bromoacetyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2β-carboxylic acid methyl ester).
[0193] To a solution of the bromo compound (17 mg, 0.053 mmol) in PBS (0.5 ml), thiolated BSA (15 mg) in PBS (0.5 ml) was added and stirring continued at ambient temperature for 3 days. The conjugate was purified by dialysis against PBS and then analyzed by mass spectral analysis.
example 2
Synthesis of PS-4
[0194] To a solution of ecgonine methyl ester (32 mg, 0.16 mmol) in DMF (2 ml), triethylamine (22 μl, 0.16 mmol), followed by succinic anhydride (16 mg, 0.16 mmol) was added and the solution heated at 35 C for 2 hours. The solvent was removed under reduced pressure and the residue purified by silica gel flash chromatography (9:1 chloroform:methanol as the eluent). This furnished the desired hemisuccinate (21 mg, 44%) as a white powder (3β-(Succinoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2β-carboxylic acid methyl ester).
[0195] To a solution of the hemisuccinate (2.4 mg, 7.69 μmol) in distilled water (0.5 ml) at 0° C., EDC (1.5 mg, 7.69 μmol) was added. After 10 minutes, BSA (2 mg in 0.5 ml PBS) and the solution allowed to warm to ambient temperature overnight. The conjugate was purified by dialysis against PBS and the degree of haptenation determined by mass spectral analysis.
example 3
Synthesis of PS-5
[0196] Method A
[0197] A solution of norcocaine hydrochloride (1 g, 3.07 mmol), triethylamine (0.86 ml, 6.14 mmol) in methylene chloride (20 ml) was treated with succinic anhydride (614 mg, 6.14 mmol) and the mixture heated at 45° C. overnight. The solvents were removed under reduced pressure and the residue purified using silica gel flash chromatography (2:1 chloroform:methanol as the eluent) This gave succinylated norcocaine (1.0 g, 84%) as a thick syrup (3β-(Benzoyloxy)-8-succinoyl-8-azabicyclo[3.2.1]octane-2β-carboxylic acid methyl ester).
[0198] To a solution of the acid (14 mg, 0.036 mmol) in distilled water (1 ml) at 0° C., EDC (10.4 mg, 0.055 mmol) was added. After 5 minutes a solution of BSA (14 mg) in PBS (1 ml) was added dropwise and the mixture allowed to warm to ambient temperature overnight. The conjugate was purified by dialysis against PBS and the degree of conjugation analyzed by mass spectral analysis.
[0199] Method B
[0200] To a solution of BSA (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current | aaaaa | aaaaa |
| Digital information | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com