Novel fusidic acid derivatives
A technology of fusidic acid, compounds, applied in the field of new fusidic acid derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0281] THF = Tetrahydrofuran
[0282] TLC = Thin Layer Chromatography
[0283] rt = room temperature
[0284] Saturated NaCl = saturated aqueous sodium chloride solution
[0285] TMS = Trimethylsilyl
[0286] v = volume
[0287] Preparation of compounds of the present invention
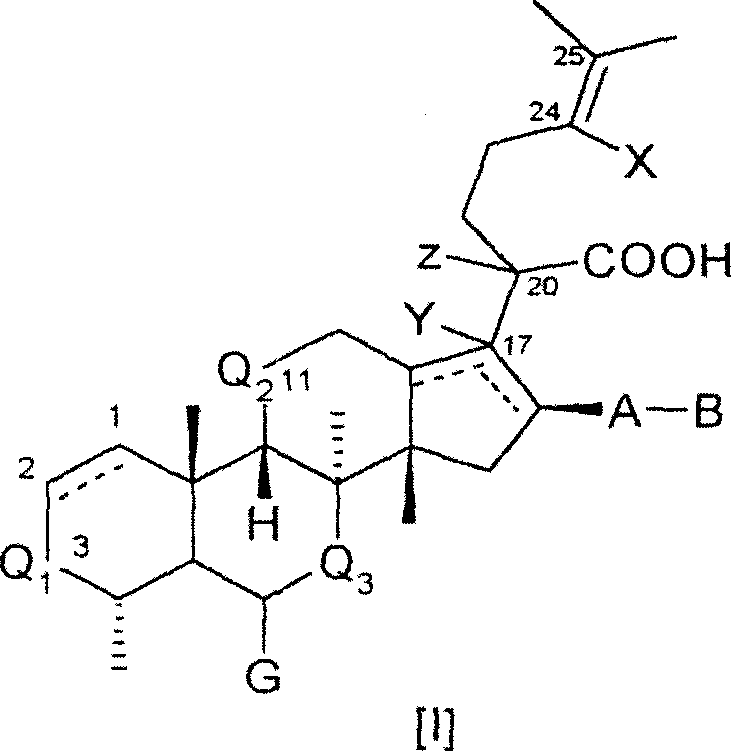
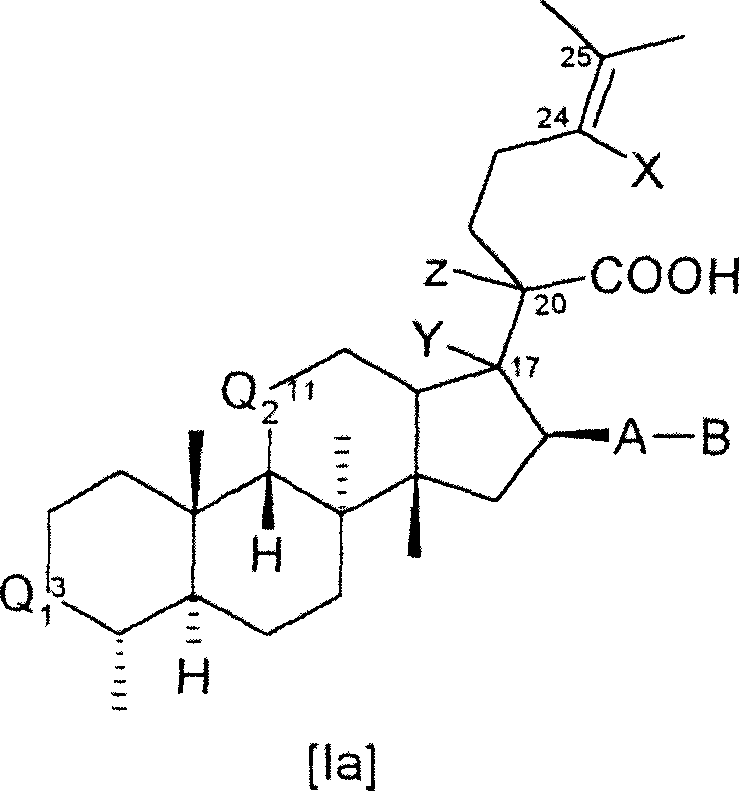
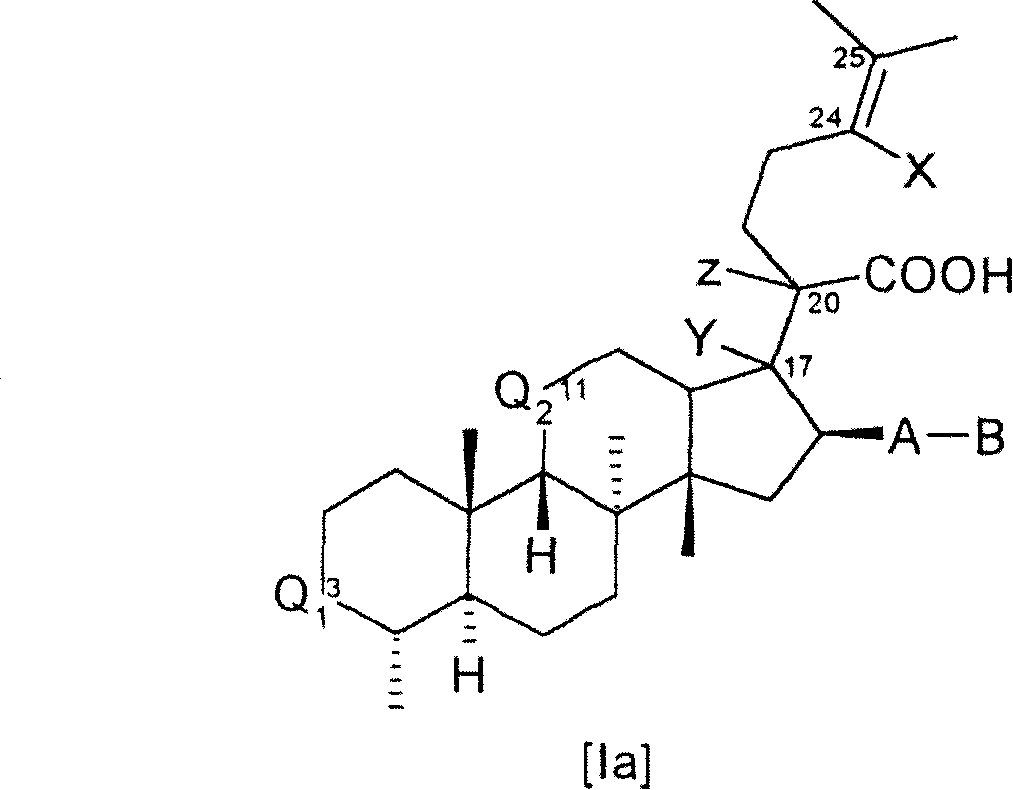
[0288] Compounds of formula I can be synthesized from known starting materials by different synthetic routes, depending on the requirements presented by each individual compound I, such as the availability of starting materials, temporary protection of sensitive substituents, the purity and yield of the synthetic steps and the number of steps. Choice of preferred order.
[0289] Illustrative, non-limiting synthesis methods and examples of the different compounds of formula I or Ia are given below. Various synthetic methods can be combined with each other, as is conveniently judged by those skilled in the art, to provide the desired compound of formula I or Ia and the free acid, salt or easily hy...
Embodiment 36-43
[0309] 24-Bromo-fusidic acid - or 24-bromo-fusidic acid analog -acetoxymethyl ester or -pivaloyloxymethyl ester can be hydrolyzed to the corresponding free acid, e.g. by using methanol and base Aqueous treatment. Heating the bromo-acid with copper(I) iodide and potassium iodide in HMPA at 120°C yields the corresponding 24-iodic acid of formula I. The acid was esterified to the corresponding phenacyl ester by treatment with phenacyl bromide and potassium fluoride in DMF. The phenacyl ester yields the corresponding 24-trifluoromethyl ester, for example by reaction with trifluoromethylcopper in HMPA. The ester is finally converted to free 24-trifluoromethylfusidic acid (or a fusidic acid analogue) of formula I by hydrolysis, such as base hydrolysis.
[0310] Alternatively, 24-iodic acids can be esterified to their acetate or pivaloyloxymethyl esters as described above, and they can be converted to the corresponding 24-aryl or alkene by a suitable coupling reaction yl esters et...
preparation example 1
[0338] Preparation Example 1: Acetoxymethyl Fusidate (2a)
[0339] Will Et 3 N (45ml; 33g; 320mmol) was added to a solution of fusidic acid (201) (128.6g; 250mmol) in DMF (375ml), and the mixture was stirred at room temperature for 30 minutes. Chloromethyl acetate (49ml; 55g; 500mmol) was then added and the reaction mixture was stirred at room temperature overnight before being worked up (EtOAc, water) to yield a crude product. The crude ester (2a) was crystallized from isopropyl ether to give pure compound (2a) as a colorless powder, m.p.103-105°C
[0340] 13 C NMR, (CDCl 3 ): 170.4, 169.6, 168.4, 150.6, 132.7, 129.3, 122.9, 79.4, 74.4, 71.4, 68.2, 49.2, 48.7, 44.3, 39.5, 39.0, 37.1, 36.2, 36.2, 35.5, 32.4, 30.3, 30.0, 28 28.3, 25.7, 24.2, 22.8, 20.9, 20.8, 20.7, 17.9, 17.7, 15.9
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com