Recombinant solvent protein, its production and use
A technology of fusion protein and recombinant protein, which is applied in beverage, food, biological, and pharmaceutical fields, and can solve the problems of increasing resistance, side effects, high incidence, and the preparation method and application of recombinant fusion protein immunosuppressants have not been found yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0268] Embodiment 1, the preparation method of hICOS-IgG1
[0269] Sources of main reagents:
[0270] Lipofectamine2000 and TRIZOL reagents were purchased from Invitrogen; various tool enzymes were purchased from Takara; hygromycin was purchased from Shanghai Pufei Biotechnology Company; DNA purification and recovery kits and plasmid DNA extraction kits were purchased from Vertejie; IL-2 , IFN-γELISA detection kit was purchased from Jingmei Biotechnology Company. The pMD-18T cloning vector was purchased from TAKARA Company, the pSecTag2 / Hygro A expression vector was purchased from Invitrogen Company, the pGL-3-Basic cloning vector, and the plasmid pMD-18T-Ig carrying the Fc gene of immunoglobulin were preserved in our laboratory; serum-free Medium EX302 was purchased from JRH Company of the United States; protein A affinity purification kit was purchased from Sigma Company of the United States; 293 cells were purchased from Institute of Cells, Chinese Academy of Sciences.
...
Embodiment 2
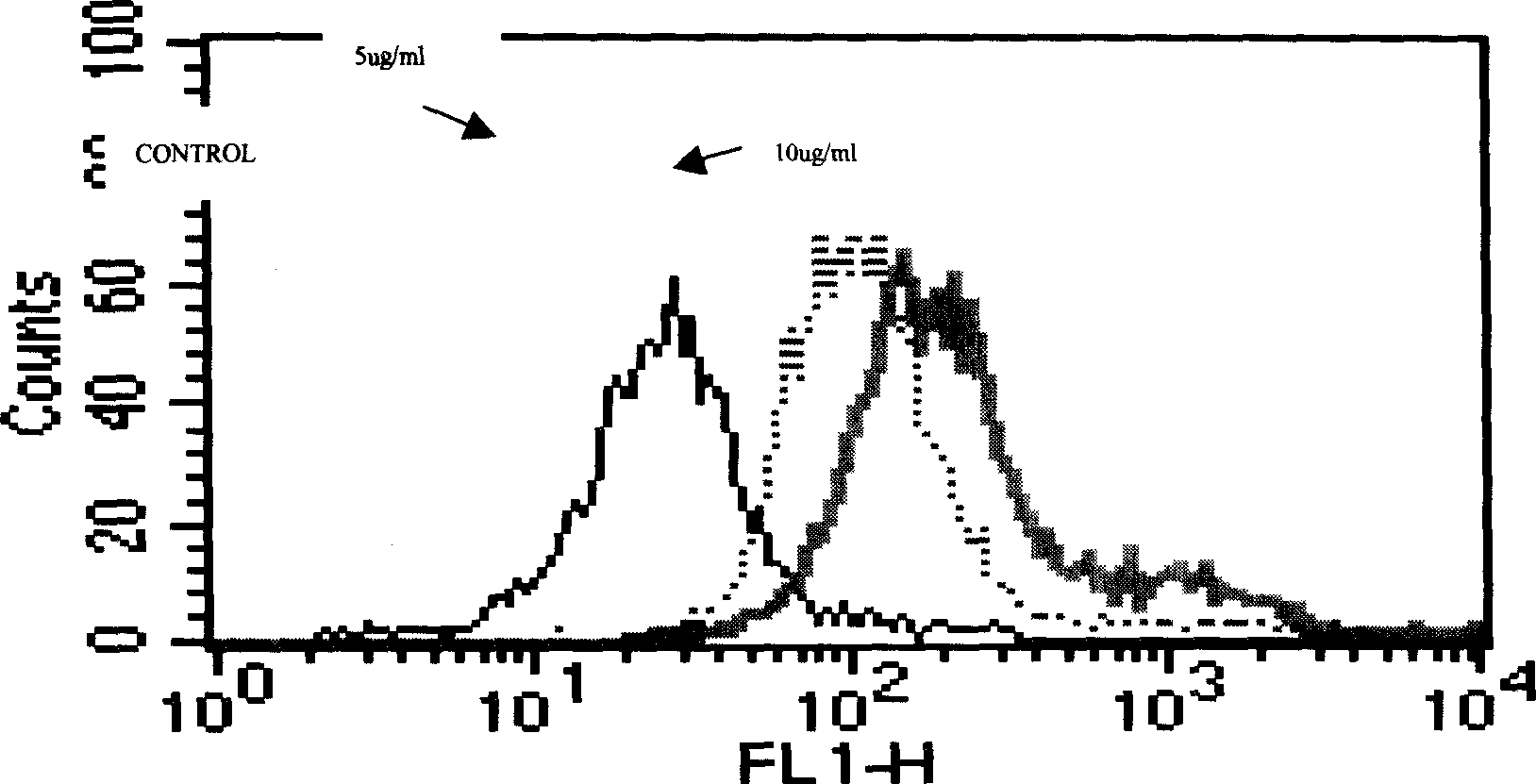
[0295] Embodiment 2, hICOS-IgG1 and ICOS ligand binding test
[0296] (1) Principle
[0297] hICOS-IgG1 can specifically bind to the ICOS ligand on the surface of its ligand-expressing cells. When the amount of ligand is constant, the amount of hICOS-IgG1 binding to its ligand increases with the increase of hICOS-IgG1 content. The fluorescently labeled anti-IgGFc antibody can be combined with the ligand-bound hICOS-IgG1, and the fluorescence emitted by the bound anti-IgGFc antibody can be detected by flow cytometry to reflect the combination of hICOS-IgG1 and its ligand.
[0298] (2) Operation steps
[0299] After blocking the FcR of Daudi cells with 20% rabbit serum, take 1×10 Daudi cells 6 After washing twice with PBS, add 2ug / ml, 10ug / ml two concentrations of hICOS-IgG1 and blank control respectively, incubate at 37°C for 1 hour, use HRP-labeled anti-human IgG as the detection antibody, and use flow cytometry Assay for ligand binding activity.
[0300] (3) Results
[0...
Embodiment 3
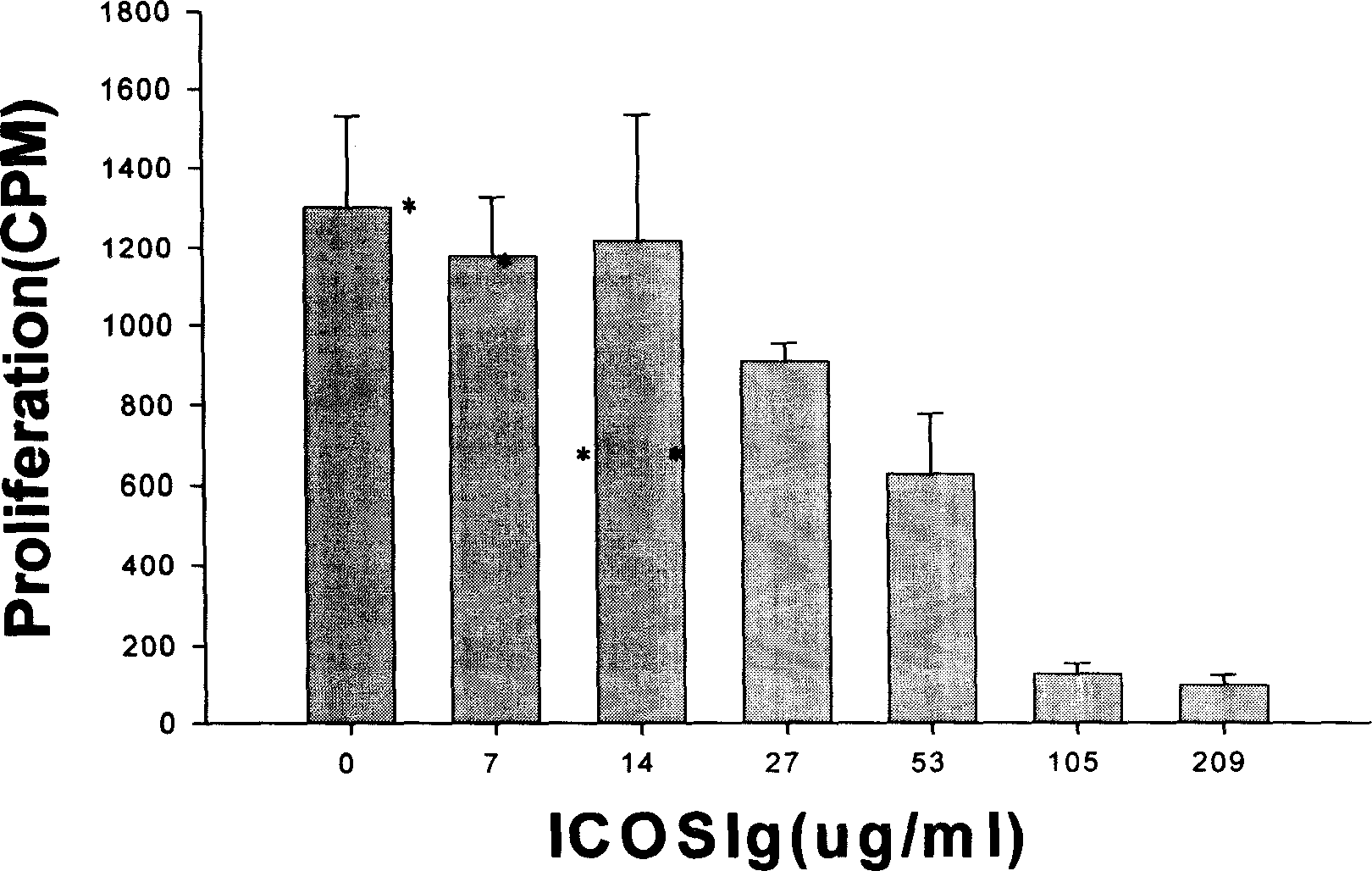
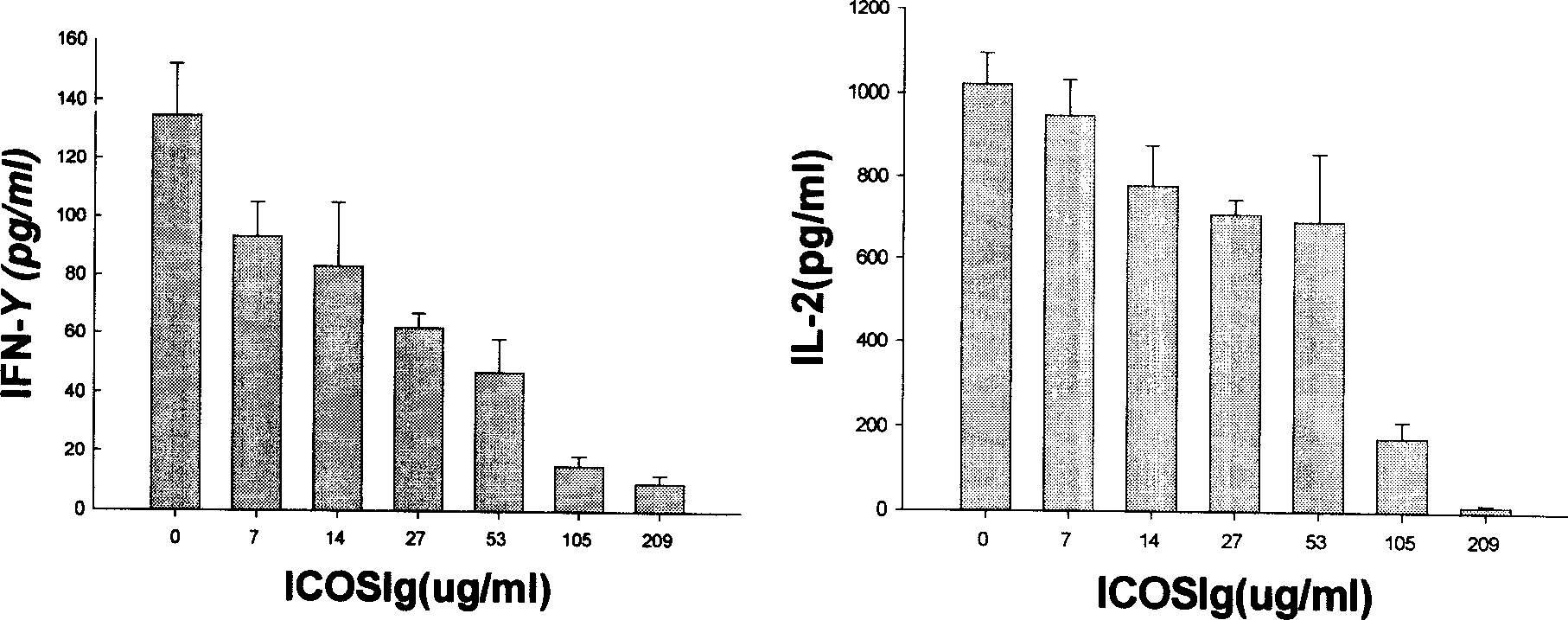
[0302] Embodiment 3, hICOS-IgG1 biological activity assay
[0303] (1) Principle
[0304] After lymphocytes are stimulated by antigens, with the participation of co-stimulatory molecules, lymphocytes are activated and exhibit biological activities such as proliferation and secretion of cytokines. Without the participation of ICOS co-stimulatory molecules, the activated lymphocytes will quickly inactivate and even apoptotic. Adding hICOS-IgG1 in the process of allogeneic lymphocyte interaction, if lymphocyte proliferation is inhibited or cytokine secretion is inhibited, it reflects that the co-stimulatory pathway of ICOS is blocked, indicating that hICOS-IgG1 has the ability to block the ICOS co-stimulatory pathway and can inhibit the proliferative response between allogeneic lymphocytes.
[0305] (2) Operation steps
[0306] Peripheral blood mononuclear cells (2×10 6 / ml) each 50ul, add hICOS-IgG1 100ul (final concentration is 209,105,53,27,14,7,0ug / ml) that contains the 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com