Medicinal composition for treating cerebrovascular disease
A composition and cardiovascular technology, applied in cardiovascular system diseases, drug combination, drug delivery, etc., can solve problems such as affecting coronary perfusion, accelerating myocardial cell apoptosis, and no therapeutic effect, and achieving low therapeutically effective doses, reducing Myocardial oxygen consumption, reduction of angioedema and dry cough
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
[0051] According to the following formula, it is pressed with the production process of the tablet, and the dispersible tablet meeting the quality standard is obtained:
[0052] recipe l
[0053] Bisoprolol Fumarate 3g Candesartan Medoxil 4g
[0054] Microcrystalline cellulose l00g cross-linked PVP 60g
[0055] Poloxamer 9g PVP 3g
[0056] Ethanol 80ml Magnesium stearate 1.2g
[0057] 180.2g
[0058] Made into 1000 pieces, each weighing 0.18g
[0059] Recipe 2
[0060] Bisoprolol Fumarate 2.5g Candesartan Medoxil 3.5g
[0061] Microcrystalline Cellulose 120g Cross-linked PVP 50g
[0062] Poloxamer 9g PVP 3g
[0063] Ethanol 80ml Magnesium stearate 1.2g
[0064] 189.2g
[0065] Made into 1000 pieces, each weighing 0.19g
[0066] Recipe 3
[0067] Bisoprolol Fumarate 4g Candesartan Medoxil 3g
[0068] Microcrystalline Cellulose 120g Cro...
Embodiment 2
[0094]Embodiment 2: the acute toxicity research of compound preparation
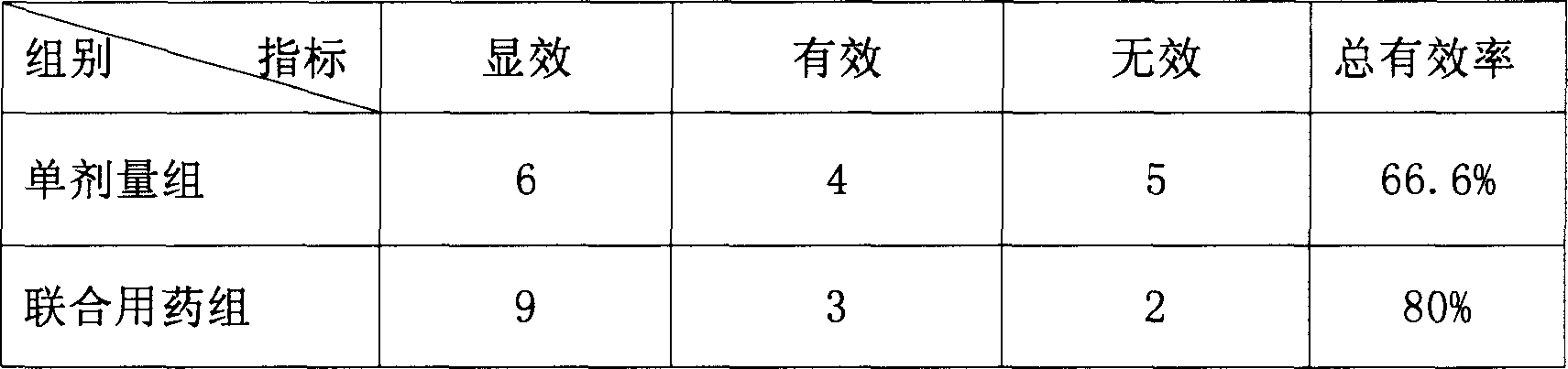
[0095] Test drug: compound dispersible tablet containing 3 mg of bisoprolol fumarate (equivalent to 2.5 mg of bisoprolol) and 4.2 mg of candesartan cilexetil (equivalent to 3 mg of candesartan)
[0096] High dose: each rabbit is administered once a day, and the dosage is equivalent to 5.76mg of the drug ingredient, with an average of 2.4mg / kg, which is 20 times the dosage for humans;
[0097] Medium dose: each rabbit is administered once a day, and the dosage is equivalent to 2.88mg of the drug ingredient, with an average of 1.2mg / kg, which is 9.5 times the dosage for humans;
[0098] Low dose: each rabbit is administered once a day, and the dose is equivalent to 1.44 mg of the drug ingredient, with an average of 1.2 mg / kg, which is 3.8 times the human dose;
[0099] Negative control: adjuvant blank tablet of compound dispersible tablet, which does not contain drug ingredients,
[0100] Animals: rabbit...
Embodiment 3
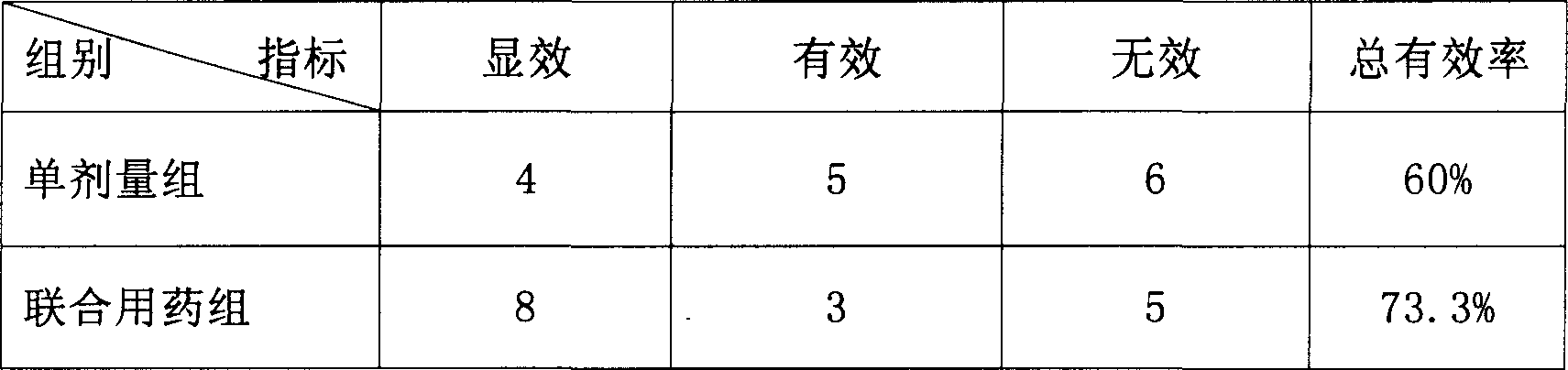
[0107] Embodiment 3: the long-term toxicity study of the topical administration of compound preparation
[0108] Test drug: same as embodiment 2
[0109] High dose: Each rat is given a single dose of 1.152 mg of drug ingredients, with an average of 4.8 mg / kg, which is 40 times the amount used by humans;
[0110] Medium dose: Each rat is administered once with an equivalent of 0.576mg of the drug ingredient, with an average of 2.4mg / kg, which is 20 times the dosage for humans;
[0111] Low dose: Each rat is administered once with an equivalent of 0.288 mg of the drug ingredient, with an average of 1.2 mg / kg, which is 10 times the amount used by humans;
[0112] The weight of rats is 0.236~0.264kg
[0113] Three doses were administered once a day, observed continuously for 30 days, and repeated the test three times. As a result, the whole body of the animal had no obvious changes; the anatomical observations were executed by dislocation, and there were no significant changes i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com