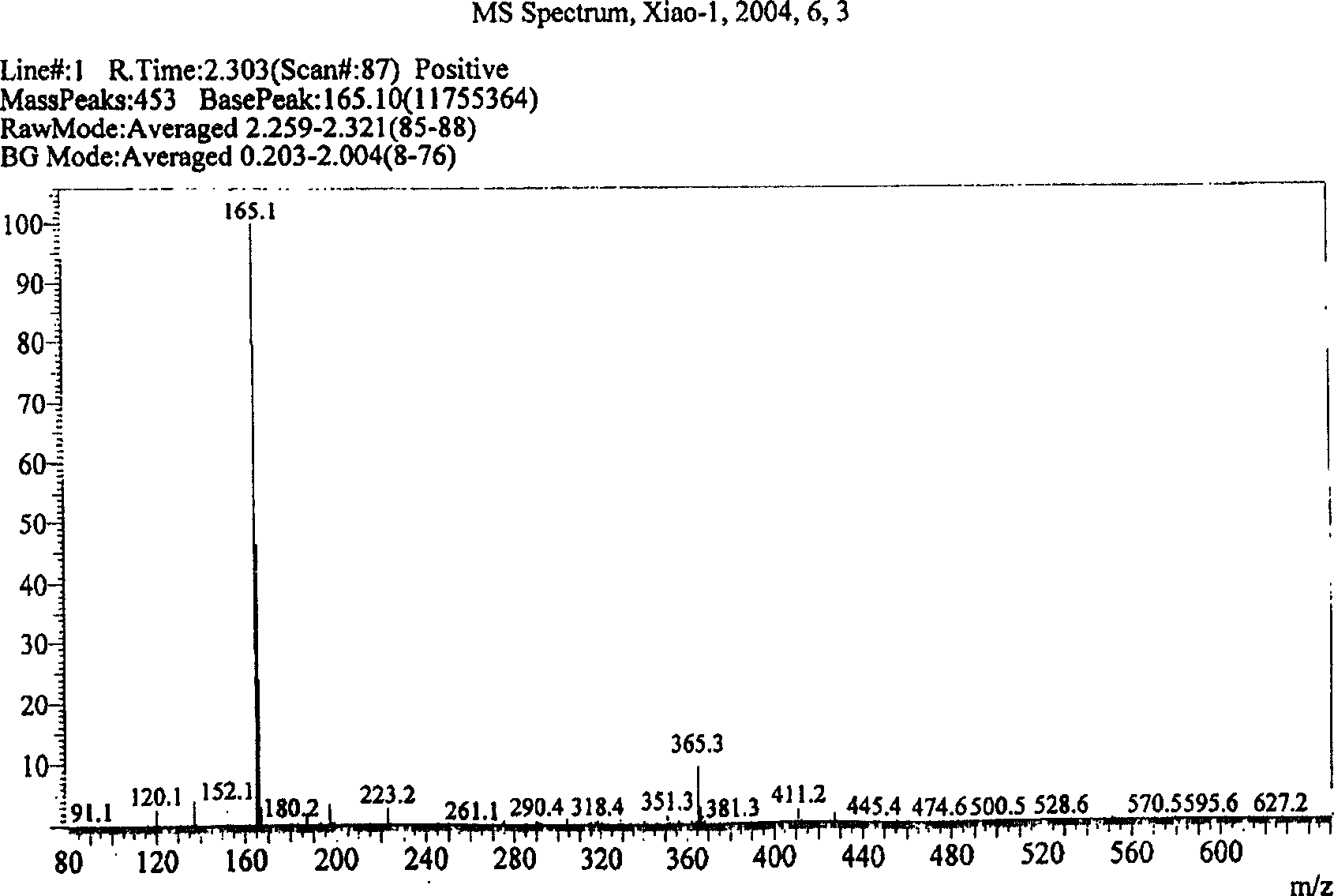
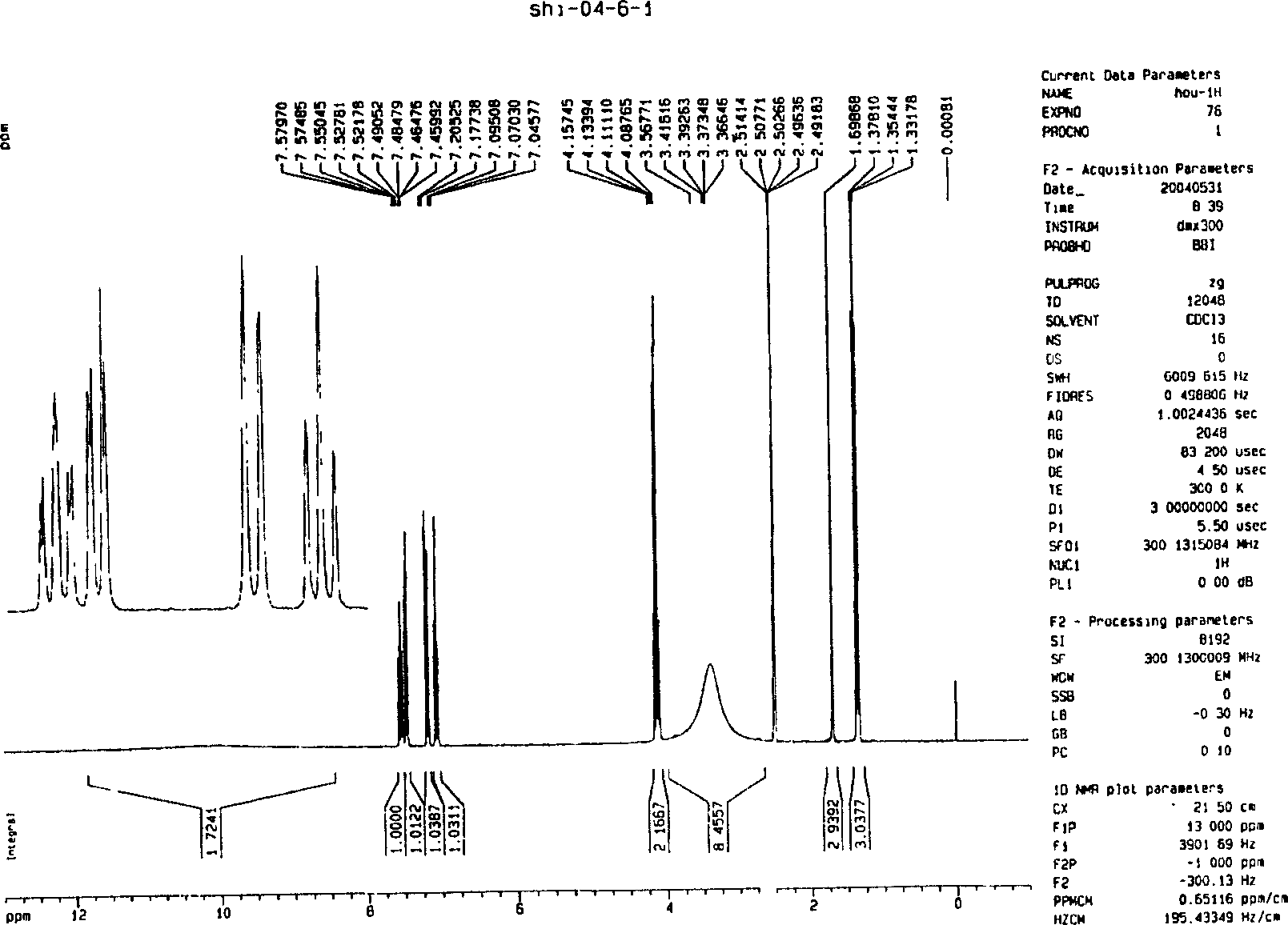
Method for synthesizing O-ethoxy phenyl formamidine acetate
A technology of o-ethoxybenzamidine and ethoxybenzamidine, which is applied in the field of pharmaceutical intermediate synthesis, can solve the problem of high cost and achieve the effects of low cost, simple post-processing and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
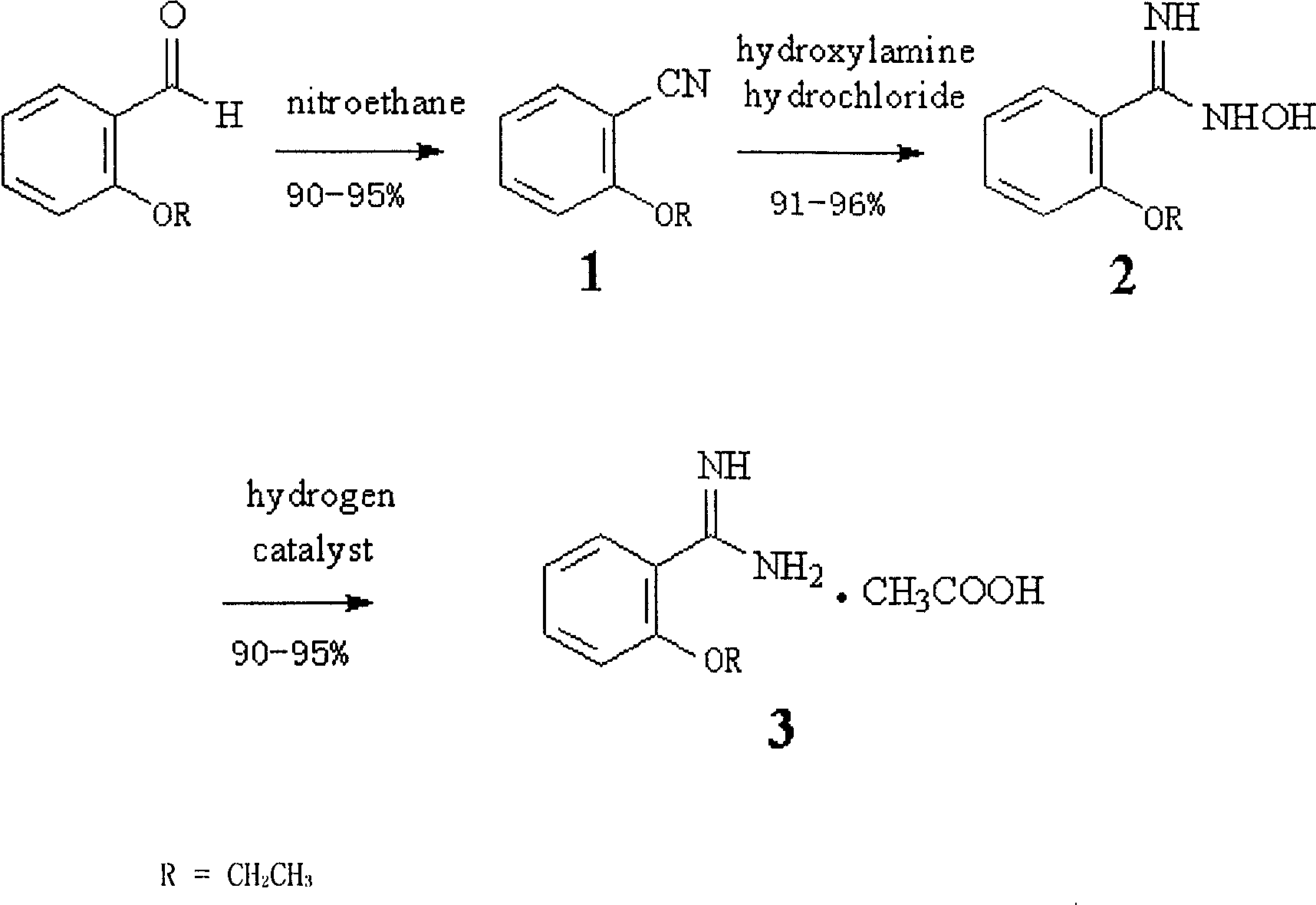
[0027] Take 7.5 grams (0.05mol) of o-ethoxybenzaldehyde, 4.31 grams (0.0575mol) of nitroethane and 6.64 grams (0.0575mol) of anhydrous pyridine hydrochloride in a container, heat to 114 ° C, and reflux for 1 hour , after cooling to room temperature, add 100 ml of chloroform and 100 ml of 0.1M hydrochloric acid solution, mix thoroughly and separate the organic layer, extract the aqueous phase with chloroform 3 times, 20 ml each time, combine the organic phases and wash with water three times, each time 50ml. The product was separated through a silica gel column with a length of 6 cm and a diameter of 2.5 cm and chloroform as eluent to obtain o-ethoxybenzonitrile with a yield of 92%.
[0028] Add 15 ml of ethanol to 5.9 g (0.04 mol) of o-ethoxybenzonitrile, 4.2 g (0.06 mol) of hydroxylamine hydrochloride, and 4.2 g of potassium carbonate, add 15 ml of water in batches under stirring, and heat to 78°C after no bubbles are generated. , refluxed for 3 hours, distilled off ethanol,...
Embodiment 2
[0031] Take 7.5 grams (0.05mol) of o-ethoxybenzaldehyde, 4.31 grams (0.0575mol) of nitroethane and 6.64 grams (0.0575mol) of anhydrous pyridine hydrochloride in a container, heat to 114 ° C, and reflux for 0.5 hours , after cooling to room temperature, add 100 ml of chloroform and 100 ml of 0.1M hydrochloric acid solution, mix thoroughly and separate the organic layer, extract the aqueous phase with chloroform 3 times, 20 ml each time, combine the organic phases and wash with water three times, each time 50ml. The product was separated through a silica gel column with a length of 6 cm and a diameter of 2.5 cm and chloroform as eluent to obtain o-ethoxybenzonitrile with a yield of 92%.
[0032] Add 15 ml of ethanol to 5.9 g (0.04 mol) of o-ethoxybenzonitrile, 4.2 g (0.06 mol) of hydroxylamine hydrochloride, and 4.2 g of potassium carbonate, add 15 ml of water in batches under stirring, and heat to 78°C after no bubbles are generated. , refluxed for 5 hours, distilled off ethan...
Embodiment 3
[0035] Take 7.5 grams (0.05mol) of o-ethoxybenzaldehyde, 4.31 grams (0.0575mol) of nitroethane and 6.64 grams (0.0575mol) of anhydrous pyridine hydrochloride in a container, heat to 114 ° C, and reflux for 2 hours , after cooling to room temperature, add 100 ml of chloroform and 100 ml of 0.1M hydrochloric acid solution, mix thoroughly and separate the organic layer, extract the aqueous phase with chloroform 3 times, 20 ml each time, combine the organic phases and wash with water three times, each time 50ml. The product was separated through a silica gel column with a length of 6 cm and a diameter of 2.5 cm and chloroform as eluent to obtain o-ethoxybenzonitrile with a yield of 92%.
[0036] Add 15 ml of ethanol to 5.9 g (0.04 mol) of o-ethoxybenzonitrile, 4.2 g (0.06 mol) of hydroxylamine hydrochloride, and 4.2 g of potassium carbonate, add 15 ml of water in batches under stirring, and heat to 78°C after no bubbles are generated. , refluxed for 1 hour, distilled off ethanol,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com