Tanshinone I derivatives and pharmaceutical application thereof
A technology for tanshinone and derivatives, applied in the field of medicine, can solve the problems of insufficient development of the medicinal value of tanshinone I, difficult structural modification of the molecular structure, etc. water soluble effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
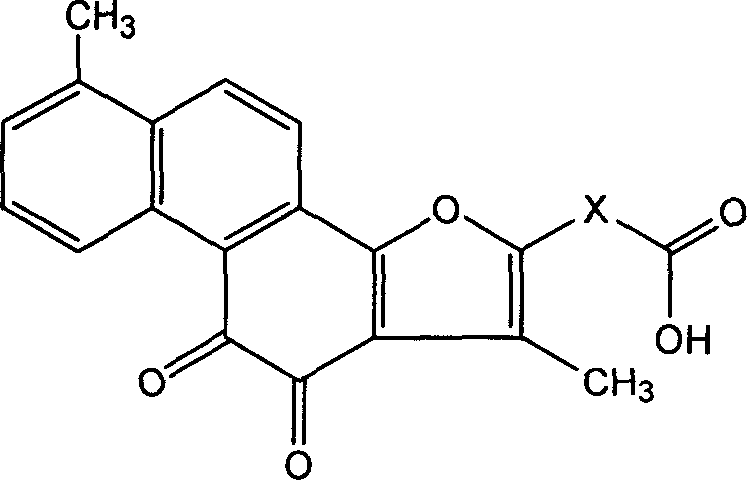
[0020] Example 1. Hydrogen spectrum, mass spectrum and analysis of 2-formaldehyde-tanshinone I and 2-acrylic acid-tanshinone I after derivatization of tanshinone I.
[0021] 2-Formaldehyde-tanshinone I
[0022] MS m / z (%): 304 (M + ), 289 (M + -CH 3 )
[0023] 1 H-NMR (CDCl 3 ,) δ9.87(s, 1H), 9.25(d, 1H), 8.26(d, 1H), 7.98(d, 1H), 7.52(q, 1H), 7.32(d, 1H), 3.71(s, 2H), 2.65(s, 3H), 2.28(s, 3H).
[0024] 2-Acrylic acid-tanshinone I:
[0025] MS m / z(%): 346(M), 331(M + -CH 3 )
[0026] 1 H-NMR (DMSO-d 6 ,) δ12.63(s, 1H), 9.21(d, 1H), 8.32(d, 1H), 8.05(d, 1H), 7.61(q, 1H), 7.39(d, 1H), 7.22(d, 1H), 6.44(d, 1H), 3.65(s, 2H), 2.52(s, 3H), 2.15(s, 3H).
Embodiment 3
[0029] The synthesis of embodiment 3.2-sodium acrylate-tanshinone I:
[0030] (1). Synthesis of 2-formaldehyde-tanshinone I: Dissolve 6.93g tanshinone I in 150mL DMF, add 20mL phosphorus oxychloride dropwise at room temperature, stir for 2 hours, pour the reaction solution into 2000mL ice water, and a solid precipitates , filtered, the filter cake was washed to neutrality, dried, and the yield was quantified.
[0031] (2). Synthesis of 2-acrylic acid-tanshinone I: Dissolve 500mg of 2-formaldehyde-tanshinone I in 100mL of benzene, add 10mL of pyridine and 240mg of malonic acid in turn, heat to reflux to separate water, react for 10 hours, evaporate under reduced pressure Remove the solvent, add 50 mL of 5% sodium carbonate aqueous solution to the residue, filter, and adjust the pH of the filtrate to nearly 2 with concentrated hydrochloric acid, then a solid is precipitated, filter and dry to obtain a solid.
[0032] (3). Synthesis of 2-sodium acrylate-tanshinone I: 500mg 2-acr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com